Protocol Template
Protocol Template - There are three templates to be used for observational research: Sections and text that are in regular font and that have not been highlighted in grey represent standard language. Find protocol templates and forms for biomedical and social behavioral research at northwestern university. Protocol templates and guidelines broadening / modernizing eligibility criteria. The following resources offer templates for authors to develop a systematic review protocol. Learn how to use the template and the. If you have any questions regarding the use of templates, please email or call the arc. The intervention template is ich gcp. A protocol template for a phase iv study of secukinumab in adult patients with psoriatic arthritis. Please use the research involving secondary use of data, documents, records or specimens template found below if your project is limited to secondary data analysis. New universal protocol template for iacuc submissions by: Amended clinical trial protocol version no. The following resources offer templates for authors to develop a systematic review protocol. If you have any questions regarding the use of templates, please email or call the arc. Find protocol templates and forms for biomedical and social behavioral research at northwestern university. There are three templates to be used for observational research: This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. Usf investigators are required to use a usf irb protocol template included in this folder. A standardized format enhances clarity and uniformity across documents. Galen cobb thursday, february 13, 2025 on january 22, the national academies of sciences,. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Amended clinical trial protocol version no. Download a microsoft word document with all 51 spirit headings and item identifiers for structuring study protocols for randomised trials. Learn how to use them, when to submit them, and what information. Phase 2 or 3 clinical trials that require. The first step in writing a protocol is to decide on the appropriate study design to address the research question. New universal protocol template for iacuc submissions by: This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. Clinical research. Protocol templates and guidelines broadening / modernizing eligibility criteria. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated studies. It contains sample text to assist. Please use the research involving secondary use of data, documents, records or specimens template found below if your project is limited to secondary. Please use the research involving secondary use of data, documents, records or specimens template found below if your project is limited to secondary data analysis. The intervention template is ich gcp. There are three templates to be used for observational research: For biomedical clinical investigations evaluating drugs and/or devices, the following templates. It contains sample text to assist investigators in. For biomedical clinical investigations evaluating drugs and/or devices, the following templates. The template includes study objectives, design, population, treatment, visit schedule,. Protocol template instructions to user: New universal protocol template for iacuc submissions by: The irb provides several protocol templates on this page. Learn how to use them, when to submit them, and what information to include in them. The first step in writing a protocol is to decide on the appropriate study design to address the research question. Amended clinical trial protocol version no. There are three templates to be used for observational research: Find protocol templates and forms for biomedical and. Learn how to use them, when to submit them, and what information to include in them. The following protocol templates are available to assist you in developing a standalone protocol: Amended clinical trial protocol version no. A standardized format enhances clarity and uniformity across documents. Using protocol templates, you can start thinking through what you need to meet compliance standards. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: For biomedical clinical investigations evaluating drugs and/or devices, the following templates. Please use the research involving secondary use of data, documents, records or specimens template found below if your project is limited to secondary data analysis. Choose a. Galen cobb thursday, february 13, 2025 on january 22, the national academies of sciences,. This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. For biomedical clinical investigations evaluating drugs and/or devices, the following templates. The irb provides several protocol templates on this page. This protocol template is a. For biomedical clinical investigations evaluating drugs and/or devices, the following templates. Clinical research is either experimental or observational. Amended clinical trial protocol version no. Phase 2 or 3 clinical trials that require. This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. Learn how to use them, when to submit them, and what information to include in them. Sections and text that are in regular font and that have not been highlighted in grey represent standard language. Download a microsoft word document with all 51 spirit headings and item identifiers for structuring study protocols for randomised trials. The following protocol templates are available to assist you in developing a standalone protocol: Using protocol templates, you can start thinking through what you need to meet compliance standards with the food and drug administration (fda) and clinical study best. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: If you have any questions regarding the use of templates, please email or call the arc. Usf investigators are required to use a usf irb protocol template included in this folder. The first step in writing a protocol is to decide on the appropriate study design to address the research question. It contains sample text to assist. Please use the research involving secondary use of data, documents, records or specimens template found below if your project is limited to secondary data analysis. Protocol template instructions to user: The irb provides several protocol templates on this page. The intervention template is ich gcp. The following resources offer templates for authors to develop a systematic review protocol. Galen cobb thursday, february 13, 2025 on january 22, the national academies of sciences,.Sales Strategy & Planning Templates Edit Online & Download
Page 176 Free Templates & Examples Edit Online & Download
FREE Protocol Templates Edit Online & Download
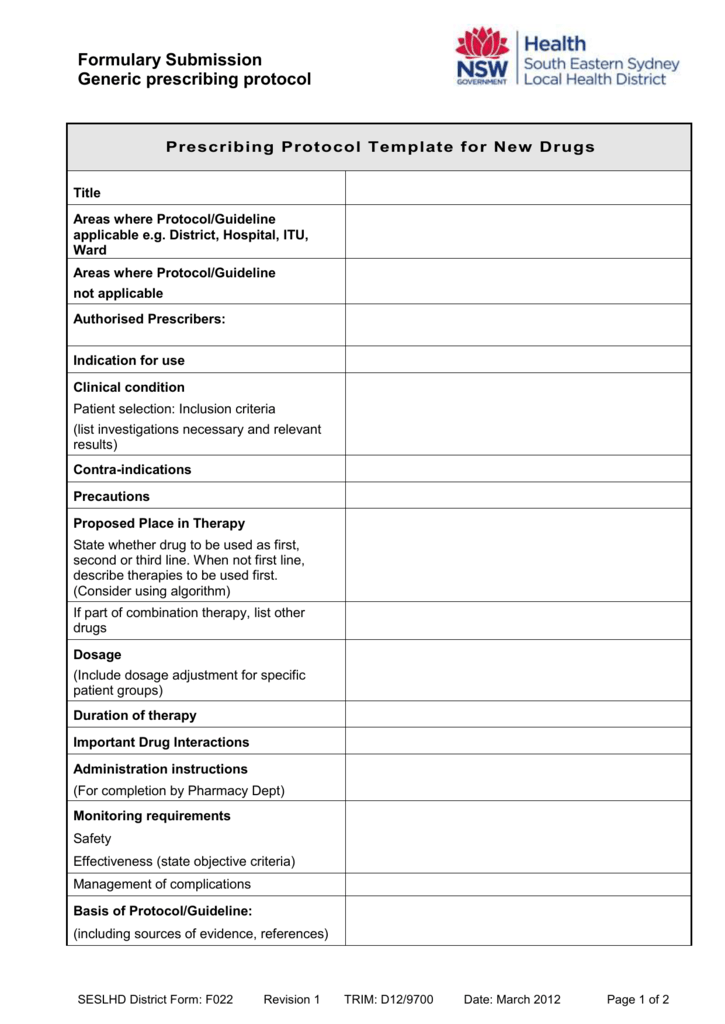
Prescribing Protocol Template for New Drugs
Minimal Risk Protocol Template INSTRUCTIONS
research protocol template
Free Trial Protocol Template Edit Online & Download
Protocol Agreement Template
Protocol Template Word
Clinical Research Protocol Template Health Care Professionals
New Universal Protocol Template For Iacuc Submissions By:
It Contains Sample Text To Assist Investigators In.
Learn How To Use The Template And The.
This Protocol Template Is A Tool To Facilitate The Development Of A Research Study Protocol Specifically Designed For The Investigator Initiated Studies.
Related Post: