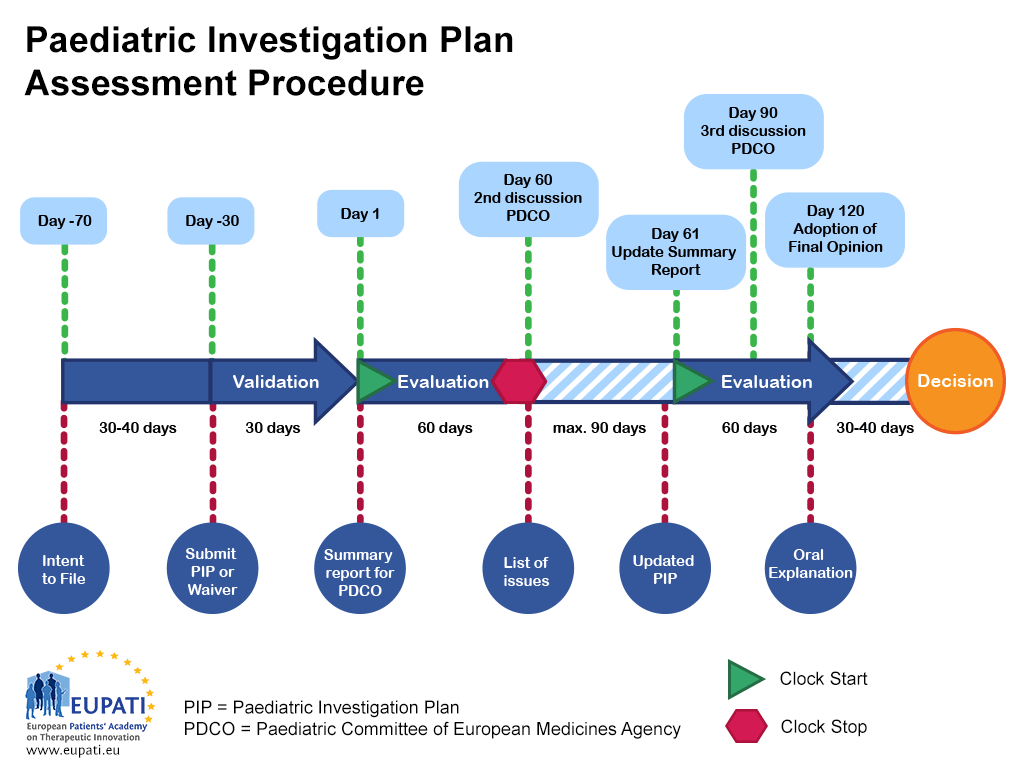
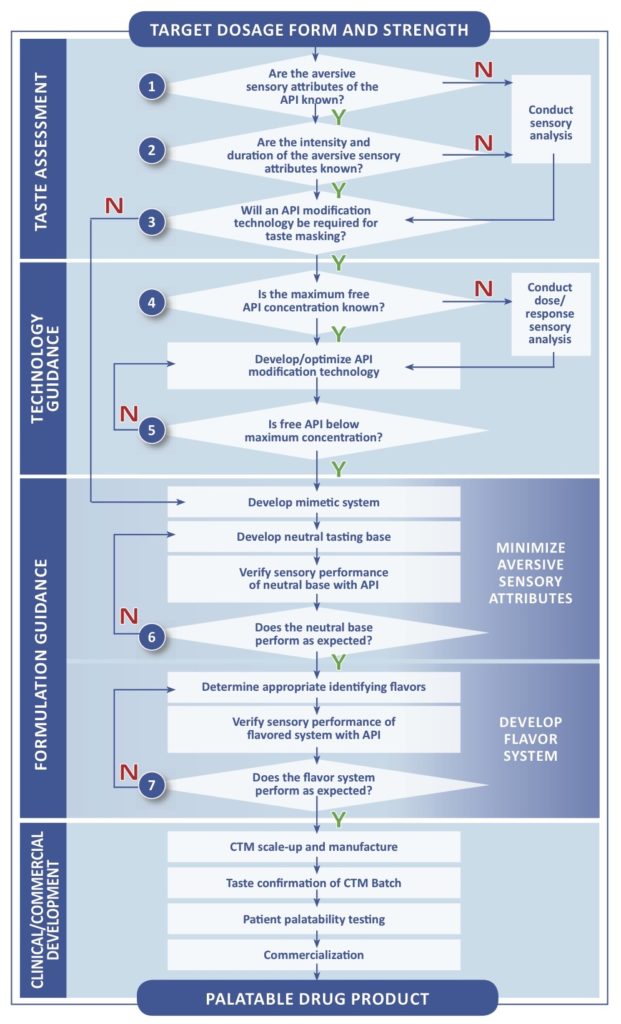
Paediatric Investigation Plan Template
Paediatric Investigation Plan Template - List of required documents by submission. Application for a paediatric investigation plan or waiver author: A document which lays out the plans for investigating the effects of a new drug or therapy on the paediatric population (infants, children and adolescents) The templates for submission and submission deadlines can be found at: According to regulation (ec) no 1901/2006, a paediatric investigation plan (pip) is a detailed research and development program of medicinal product to be authorized for treatment of. In europe, sponsors must possess a compliant paediatric investigation plan (pip) when applying for marketing approval of drugs. 1) define the pip strategy early in the writing process. Clinical studies in cases where elements cannot be defined in full, a milestone should be. European medicines agency created date: This page lists the templates and forms required by companies wishing to submit a paediatric application. European medicines agency created date: A document which lays out the plans for investigating the effects of a new drug or therapy on the paediatric population (infants, children and adolescents) A paediatric investigation plan (pip) is a research and development requirement aimed at ensuring the availability and conformity of medicines to the paediatric population in all. The core deliverable is the ‘scientific part of the application. Clinical studies in cases where elements cannot be defined in full, a milestone should be. A paediatric investigation plan is assessed by the paediatric committee of the european medicines agency and follows a set procedure with defined timelines. The forms and templates should be downloaded and saved first before. It ensures that the required. 1) define the pip strategy early in the writing process. This page lists the templates and forms required by companies wishing to submit a paediatric application. A paediatric investigation plan (pip) or pediatric study plan (psp) is a development plan intended to support the authorization of a medicine for children by ensuring data are obtained. 1) define the pip strategy early in the writing process. According to regulation (ec) no 1901/2006, a paediatric investigation plan (pip) is a detailed research and development program of medicinal product. • be prepared for uncertainties in team about paediatric requirements + appropriateness of planned measures • potential gap in awareness of european paediatric requirements between. Clinical studies in cases where elements cannot be defined in full, a milestone should be. 1) define the pip strategy early in the writing process. The core deliverable is the ‘scientific part of the application.. Complete a table for each study, and copy & paste additional tables where. Application for a paediatric investigation plan or waiver author: It streamlines the process by ensuring thorough collection of relevant information, differential diagnosis, and a tailored management plan. The timing and content of the The templates for submission and submission deadlines can be found at: A paediatric investigation plan is assessed by the paediatric committee of the european medicines agency and follows a set procedure with defined timelines. This page lists the templates and forms required by companies wishing to submit a paediatric application. • the development of paediatric regulations in the us over time • the definition, intent and timing of submission to the. The forms and templates should be downloaded and saved first before. A paediatric investigation plan is assessed by the paediatric committee of the european medicines agency and follows a set procedure with defined timelines. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the authorisation of a medicinal product for children and adolescents. In europe,. The timing and content of the 1) define the pip strategy early in the writing process. A paediatric investigation plan (pip) is a research and development requirement aimed at ensuring the availability and conformity of medicines to the paediatric population in all. Templates, forms and submission dates. In europe, sponsors must possess a compliant paediatric investigation plan (pip) when applying. Application for a paediatric investigation plan or waiver author: Templates, forms and submission dates. According to regulation (ec) no 1901/2006, a paediatric investigation plan (pip) is a detailed research and development program of medicinal product to be authorized for treatment of. A paediatric investigation plan (pip) is a research and development requirement aimed at ensuring the availability and conformity of. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the authorisation of a medicinal product for children and adolescents. In europe, sponsors must possess a compliant paediatric investigation plan (pip) when applying for marketing approval of drugs. The core deliverable is the ‘scientific part of the application. It ensures that the required. Templates, forms and. Application for a paediatric investigation plan or waiver author: 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the authorisation of a medicinal product for children and adolescents. A document which lays out the plans for investigating the effects of a new drug or therapy on the paediatric population (infants, children and adolescents) • be. The forms and templates should be downloaded and saved first before. 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the authorisation of a medicinal product for children and adolescents. It is important to carefully consider the most relevant. A paediatric investigation plan (pip) or pediatric study plan (psp) is a development plan intended to. • be prepared for uncertainties in team about paediatric requirements + appropriateness of planned measures • potential gap in awareness of european paediatric requirements between. It ensures that the required. The timing and content of the 1) define the pip strategy early in the writing process. This page lists the templates and forms required by companies wishing to submit a paediatric application. A document which lays out the plans for investigating the effects of a new drug or therapy on the paediatric population (infants, children and adolescents) 1.1.1 paediatric investigation plan (pip) a pip is a drug development plan that supports the authorisation of a medicinal product for children and adolescents. • the development of paediatric regulations in the us over time • the definition, intent and timing of submission to the fda, and types of psps • the updated template requirements included in. According to regulation (ec) no 1901/2006, a paediatric investigation plan (pip) is a detailed research and development program of medicinal product to be authorized for treatment of. Application for a paediatric investigation plan or waiver author: The forms and templates should be downloaded and saved first before. It streamlines the process by ensuring thorough collection of relevant information, differential diagnosis, and a tailored management plan. It is important to carefully consider the most relevant. In europe, sponsors must possess a compliant paediatric investigation plan (pip) when applying for marketing approval of drugs. A paediatric investigation plan (pip) or pediatric study plan (psp) is a development plan intended to support the authorization of a medicine for children by ensuring data are obtained. Templates, forms and submission dates.Paediatric medicine Paediatric Investigation Plan EUPATI
Paediatric Investigation Plan Template
Fillable Online Paediatric investigation plans Templates, forms and
Paediatric Investigation Plan Template
Pediatric Care Plan Template Medical Diagnosis Health Care
Paediatric Investigation Plan (PIP) Applications Steps to success
Paediatric Investigation Plan Template
Paediatric Investigation Plan Template
Fillable Online Paediatric investigation plans questions and answers
Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Below Are 5 Key Tips To Consider When Preparing The Pip Application.
Pediatric Studies Under Prea And Potential Pediatric Uses Under The Bpca, Is Intended To Result In A More Efficient Pediatric Drug Development Program.
A Paediatric Investigation Plan (Pip) Is A Research And Development Requirement Aimed At Ensuring The Availability And Conformity Of Medicines To The Paediatric Population In All.
The Core Deliverable Is The ‘Scientific Part Of The Application.
Related Post: