Installation Qualification Template
Installation Qualification Template - This document is a template for a process validation protocol for installation qualification. Validation document content can be. The purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. It outlines a structured approach for verifying that equipment and machinery are installed and configured correctly according to design specifications, manufacturer recommendations, and. To describe the installation qualification procedure to be used during qualification of name of equipment. It details tests to verify that the equipment is uniquely identified, installed properly,. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. A sample plan for qualifying the installation and configuration of sample software, a data replication tool, for technology workshop's disaster recovery procedure. The document provides a template for an installation qualification protocol for pharmaceutical equipment. Download a sample executed executed installation qualification. An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified,. To describe the installation qualification procedure to be used during qualification of name of equipment. Lubricants use this list to verify that the lubricants used in the. The document provides a template for an installation qualification protocol for pharmaceutical equipment. Page 8 of 17 6.5. A sample plan for qualifying the installation and configuration of sample software, a data replication tool, for technology workshop's disaster recovery procedure. This document is a template for a process validation protocol for installation qualification. The purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. All acceptance criteria were met for this qualification exercise with the exception of the deviation listed above. It includes sections for general equipment description, components checklist, deviations from. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user requirements, &. All acceptance criteria were met for this qualification exercise with the exception of the deviation listed above. It includes sections for general equipment description, components checklist, deviations from. Installation qualification for. The purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. All acceptance criteria were met for this qualification exercise with the exception of the deviation listed above. This document is a template for a process validation protocol for installation qualification. Fastval includes templates for all validation documents, including. Download a sample executed executed installation qualification. An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified,. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user requirements, &.. It details tests to verify that the equipment is uniquely identified, installed properly,. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. A sample plan for qualifying the installation and configuration of sample software, a data replication tool, for technology workshop's disaster recovery procedure. Validation document content can be.. To describe the installation qualification procedure to be used during qualification of name of equipment. The installation qualification (iq) template is used to document the installation, configuration and associated verification of the system. Template for installation qualification protocol. The purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet.. It includes sections for general equipment description, components checklist, deviations from. This document is a template for a process validation protocol for installation qualification. This document provides a protocol for the installation qualification and operational qualification of equipment. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. Installation qualification. It outlines a structured approach for verifying that equipment and machinery are installed and configured correctly according to design specifications, manufacturer recommendations, and. An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified,. This document provides a protocol for the installation qualification and operational qualification of. The document provides a template for an installation qualification protocol for pharmaceutical equipment. It details tests to verify that the equipment is uniquely identified, installed properly,. Template for installation qualification protocol. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. It defines the testing and documentation required to ensure. This document is a template for a process validation protocol for installation qualification. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified,. Validation document content. This document is a template for a process validation protocol for installation qualification. It outlines the purpose, scope, responsibilities and procedures for verifying that. Download a sample executed executed installation qualification. It details tests to verify that the equipment is uniquely identified, installed properly,. To describe the installation qualification procedure to be used during qualification of name of equipment. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user requirements, &. Lubricants use this list to verify that the lubricants used in the. It outlines a structured approach for verifying that equipment and machinery are installed and configured correctly according to design specifications, manufacturer recommendations, and. Installation and operational qualification report template sample. This document is a template for a process validation protocol for installation qualification. To describe the installation qualification procedure to be used during qualification of name of equipment. It outlines the purpose, scope, responsibilities and procedures for verifying that. The purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified,. Define what the document is to. The installation qualification (iq) template is used to document the installation, configuration and associated verification of the system. This template serves as a baseline for what should. Page 8 of 17 6.5. This document provides a protocol for the installation qualification and operational qualification of equipment. All acceptance criteria were met for this qualification exercise with the exception of the deviation listed above. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device.TEM025 Example Installation Qualification Report Sample PDF
CIQA Installation and Operational Qualification Protocol IOQ Equipment
Installation Qualification Protocol Template
Installation Qualification Sop No 0068 Quality Assurance Business
TEM 270 Installation and Operational Qualification Protocol Template
Installation Installation Qualification Template
Installation Qualification Template
Installation Qualification Template
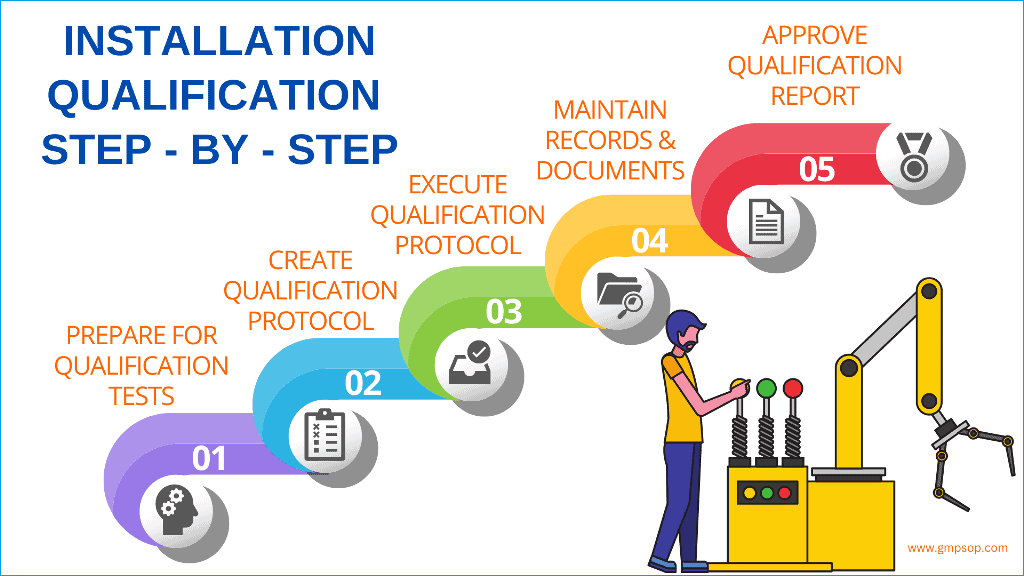
A stepbystep guide to successful installation qualification (IQ)
Installation Qualification Template
Installation Qualification For Equipment (Reference Sop:
It Includes Sections For General Equipment Description, Components Checklist, Deviations From.
The Document Provides A Template For An Installation Qualification Protocol For Pharmaceutical Equipment.
Template For Installation Qualification Protocol.
Related Post: