Free Decentralized Clinical Trials Staff Training Checklist Templates
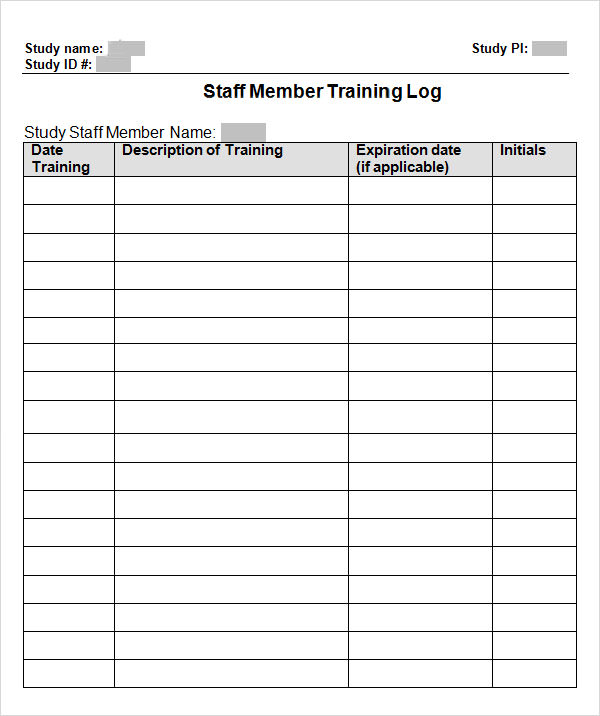
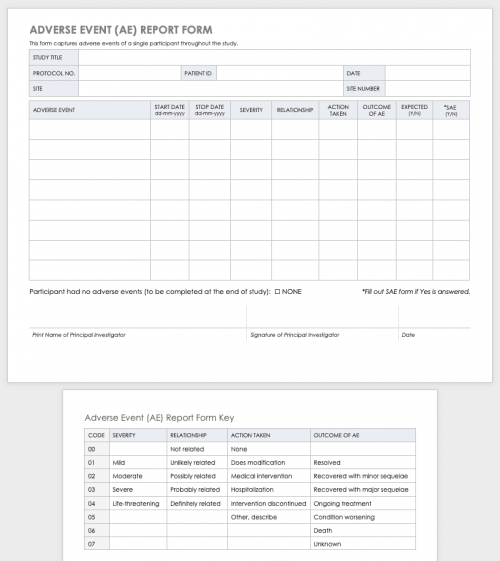
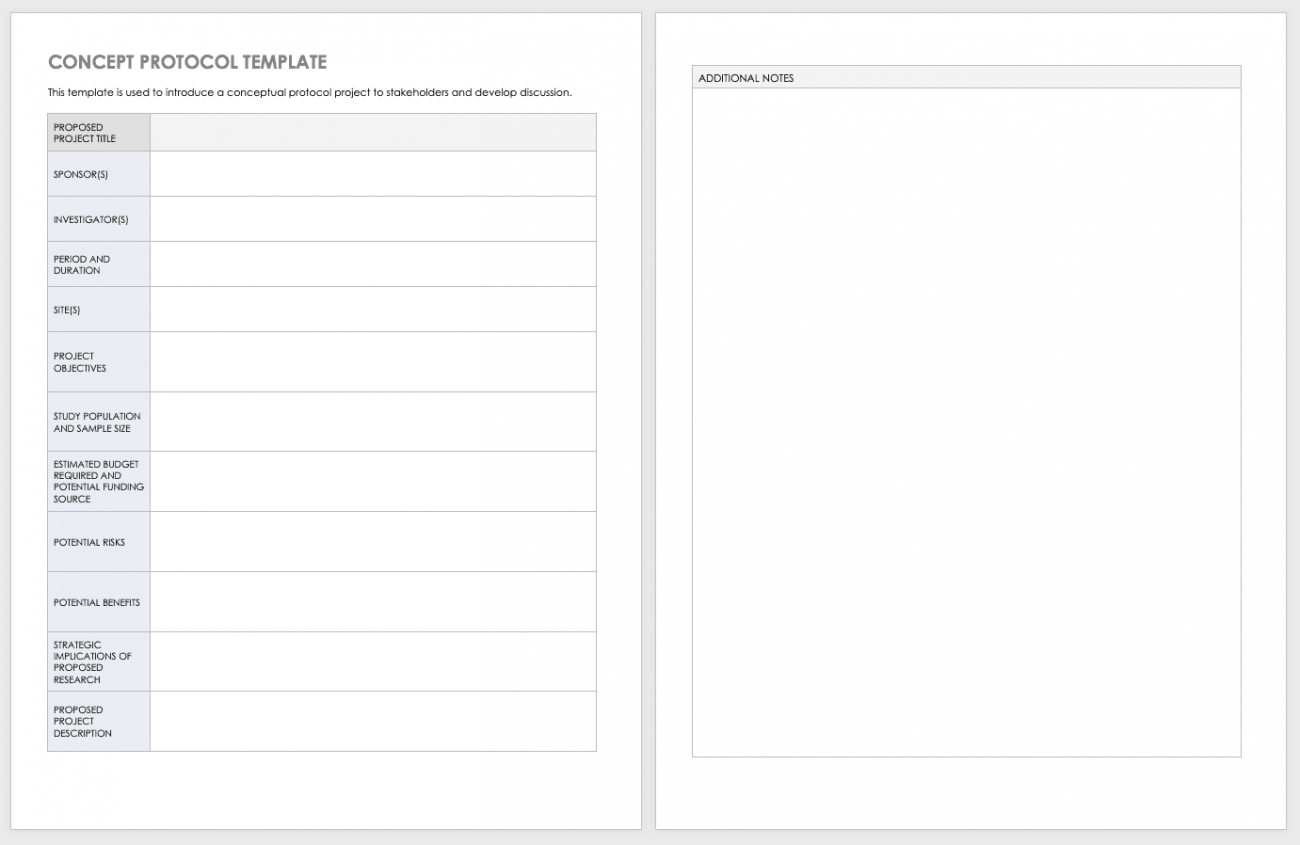
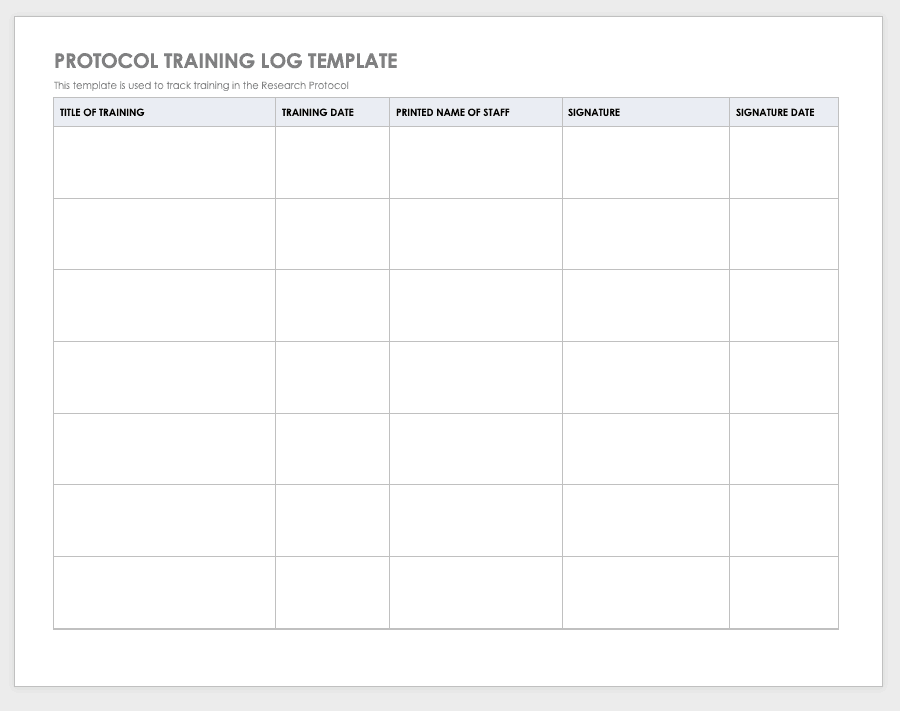
Free Decentralized Clinical Trials Staff Training Checklist Templates - Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. The focus of clinical research changes as diseases emerge and new treatments create cures for old conditions. Below, you’ll find a curated list of sop templates. It lists all the key steps, from. Commit subject matter experts (smes) to implementation workshops. How to transform and enhance decentralized clinical trials leveraging the. Sponsor, contract research organization (cro), and vendor. Each site staff member completing study activities on this trial (listed on the doa) should complete this checklist prior to beginning any work on the study. Ensure protocol adherence, patient safety, and regulatory compliance in every trial. Enhance this design & content with free ai. Effective training teaches researchers how to ethically and compliantly execute study activities, communicate with. Sponsor, contract research organization (cro), and vendor. Customize and download this clinical research coordinator training checklist. Below, you’ll find a curated list of sop templates. Use this checklist as a guide to designing your decentralized clinical trials. As diseases evolve, the ultimate goal remains to speed new. Clinical research coordinator training checklist is in editable,. We offer a mix of in person, live online, and on demand elearnings. • how does the partner ensure that its mobile nurses are following specified protocols for the clinical trial? The focus of clinical research changes as diseases emerge and new treatments create cures for old conditions. Octri offers a variety of clinical research training to the ohsu research workforce. We offer a mix of in person, live online, and on demand elearnings. The focus of clinical research changes as diseases emerge and new treatments create cures for old conditions. Below, you’ll find a curated list of sop templates. Please note that this page has been updated. Below, you’ll find a curated list of sop templates. Use this checklist as a guide to designing your decentralized clinical trials. Welcome to global health trials' tools and templates library. We offer a mix of in person, live online, and on demand elearnings. Please note that this page has been updated for 2015 following a quality check and review of. Effective training teaches researchers how to ethically and compliantly execute study activities, communicate with. Sponsor, contract research organization (cro), and vendor. Each site staff member completing study activities on this trial (listed on the doa) should complete this checklist prior to beginning any work on the study. Welcome to global health trials' tools and templates library. As diseases evolve, the. Each site staff member completing study activities on this trial (listed on the doa) should complete this checklist prior to beginning any work on the study. Use this checklist as a guide to designing your decentralized clinical trials. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new. It lists all the key steps, from. How to transform and enhance decentralized clinical trials leveraging the. Checklist for irb/ec oversight of decentralized clinical trials this document is a checklist of the issues that irbs/ecs should consider when reviewing a decentralized clinical trial. Effective training teaches researchers how to ethically and compliantly execute study activities, communicate with. • what type. Commit subject matter experts (smes) to implementation workshops. As diseases evolve, the ultimate goal remains to speed new. Sponsor, contract research organization (cro), and vendor. Welcome to global health trials' tools and templates library. Checklist for irb/ec oversight of decentralized clinical trials this document is a checklist of the issues that irbs/ecs should consider when reviewing a decentralized clinical trial. Commit subject matter experts (smes) to implementation workshops. We offer a mix of in person, live online, and on demand elearnings. • how does the partner ensure that its mobile nurses are following specified protocols for the clinical trial? Each site staff member completing study activities on this trial (listed on the doa) should complete this checklist prior to beginning. Welcome to global health trials' tools and templates library. Welcome to global health trials' tools and templates library. Octri offers a variety of clinical research training to the ohsu research workforce. Discover a comprehensive checklist for clinical trials new protocol training for study nurses. The focus of clinical research changes as diseases emerge and new treatments create cures for old. Octri offers a variety of clinical research training to the ohsu research workforce. How to transform and enhance decentralized clinical trials leveraging the. • how does the partner ensure that its mobile nurses are following specified protocols for the clinical trial? Commit subject matter experts (smes) to implementation workshops. We offer a mix of in person, live online, and on. Octri offers a variety of clinical research training to the ohsu research workforce. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Use this checklist as a guide to designing your decentralized clinical trials. Commit subject matter experts (smes) to implementation workshops. Ensure protocol adherence, patient. Here’s what to consider in adopting decentralized clinical trials. Use this checklist as a guide to designing your decentralized clinical trials. How to transform and enhance decentralized clinical trials leveraging the. It lists all the key steps, from. Below, you’ll find a curated list of sop templates. • how does the partner ensure that its mobile nurses are following specified protocols for the clinical trial? Have a ctms plan (or plans to. Octri offers a variety of clinical research training to the ohsu research workforce. Sponsor, contract research organization (cro), and vendor. Customize and download this clinical research coordinator training checklist. Welcome to global health trials' tools and templates library. Ensure protocol adherence, patient safety, and regulatory compliance in every trial. We offer a mix of in person, live online, and on demand elearnings. Commit subject matter experts (smes) to implementation workshops. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Welcome to global health trials' tools and templates library.Training Log Templates 11+ Free Printable Word, Excel & PDF
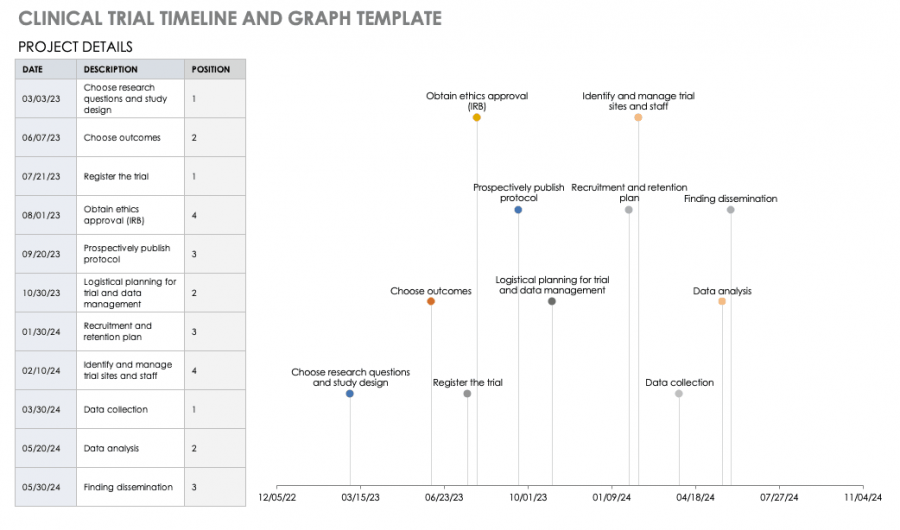
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Clinical Trial Report Template (4) TEMPLATES EXAMPLE TEMPLATES
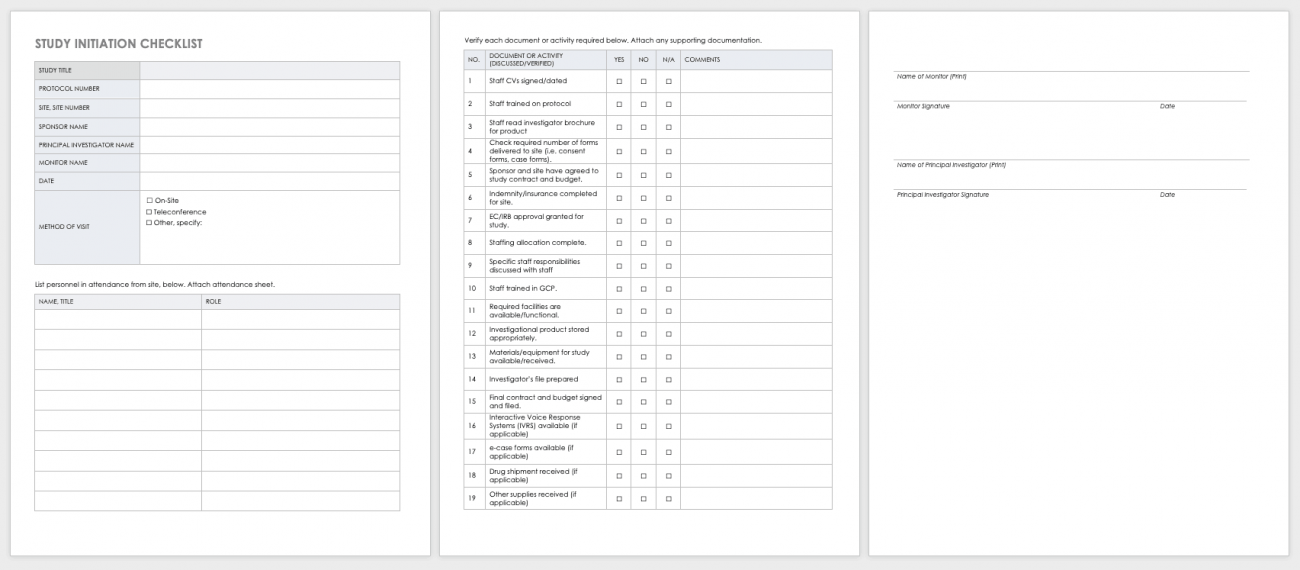
New Clinical Research Staff Orientation & Training Checklist
FREE 44+ Sample Checklist Samples & Templates in Samples in Excel PDF
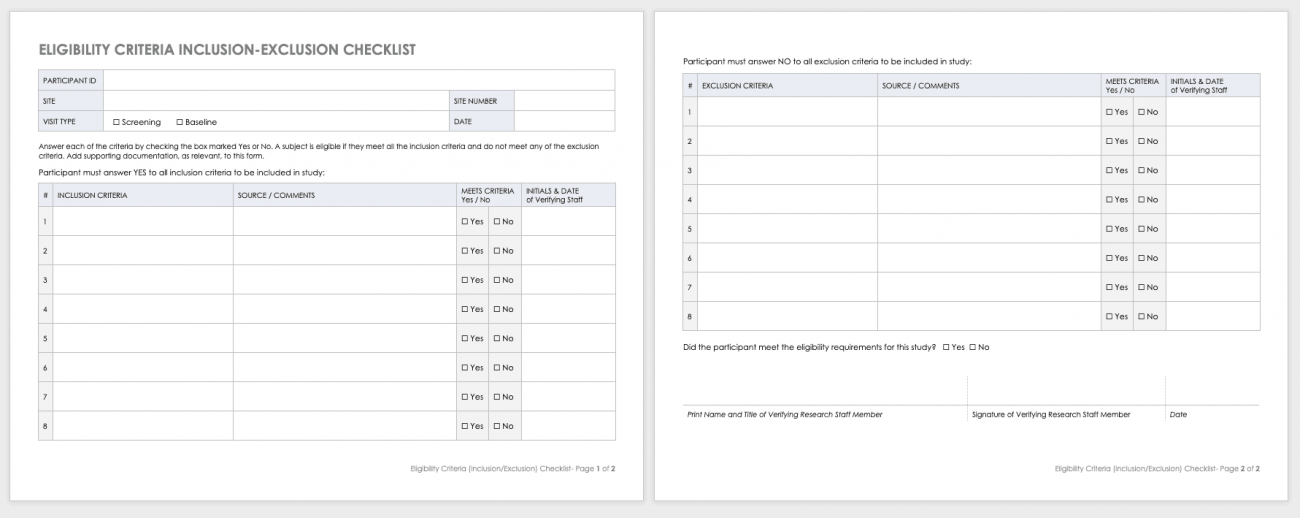
Each Site Staff Member Completing Study Activities On This Trial (Listed On The Doa) Should Complete This Checklist Prior To Beginning Any Work On The Study.
As Diseases Evolve, The Ultimate Goal Remains To Speed New.
The Focus Of Clinical Research Changes As Diseases Emerge And New Treatments Create Cures For Old Conditions.
Please Note That This Page Has Been Updated For 2015 Following A Quality Check And Review Of The Templates, And Many New Ones.
Related Post: