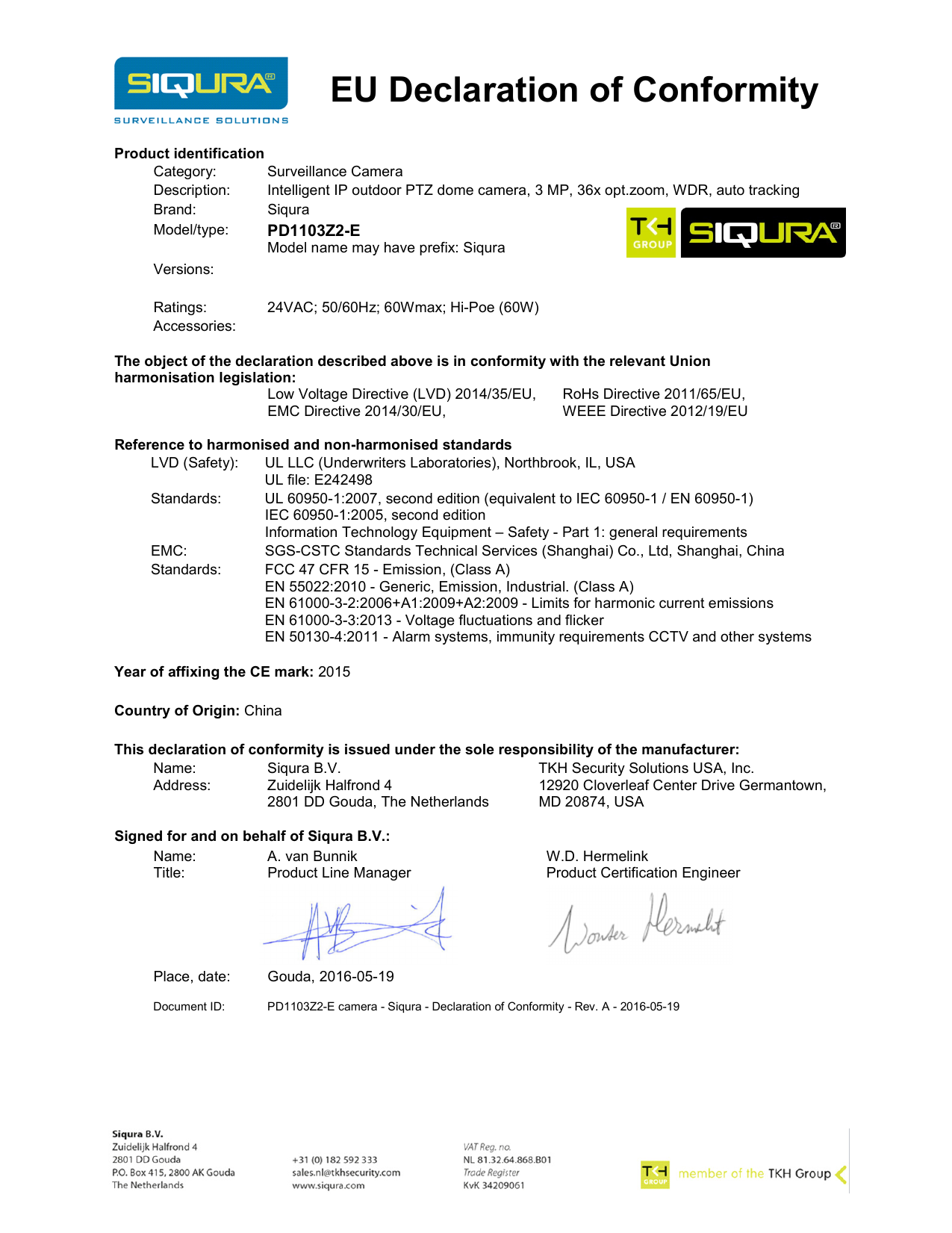
Eu Declaration Of Conformity Template
Eu Declaration Of Conformity Template - (further) the following harmonised standards and. Standards used for demonstration of compliance: These products bear the ce mark indicating conformity with the provisions of these directives. Additional information to be mentioned on the doc may be required by. Comply with medical device regulation (eu) 2017/745. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. Product model/product (product, type, batch or serial number): Essential guide to ensure compliance and meet new standards. As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu doc. Creating a declaration of conformity (doc) is an essential step for manufacturers of medical devices and in vitro diagnostic devices who wish to enter the european union market. Standards used for demonstration of compliance: How to draft the eu declaration of conformity. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu doc. This document is an eu declaration of conformity template. Product model/product (product, type, batch or serial number): Conformity assessment procedure, notified bodies, technical standards, harmonised standards, risk assessment, ce marking. The eu declaration of conformity previously was called an ‘ec declaration of conformity’. Comply with medical device regulation (eu) 2017/745. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. Additional information to be mentioned on the doc may be required by. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) Standards used for demonstration of compliance: How to draft the. Additional information to be mentioned on the doc may be required by. How to draft the eu declaration of conformity. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. Pressure equipment direc˚ve (ped) 2014/68/eu. Product model/product (product, type, batch or serial number): It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) This document is an eu declaration of conformity template. Standards used for demonstration of compliance: As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) Additional information to be mentioned on the doc may be required by. Conformity assessment procedure, notified bodies, technical standards, harmonised standards, risk assessment, ce marking. The eu declaration of conformity previously was called an ‘ec declaration of conformity’. Pressure equipment direc˚ve (ped). Standards used for demonstration of compliance: In this blog you will be guided through the process of creating a fully compliant declaration of conformity (doc), so you will be able to compile this document yourself. Product model/product (product, type, batch or serial number): Comply with medical device regulation (eu) 2017/745. This object is in conformity with the following union harmonisation. This object is in conformity with the following union harmonisation legislation: As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. Object of the declaration (identification of product allowing traceability. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation. Product model/product (product, type, batch or serial number): Essential guide to ensure compliance and meet new standards. In this blog you will be guided through the process of creating a fully compliant declaration of conformity (doc), so you will be able to compile this document yourself. Eu declaration of conformity (sample) 1. Comply with medical device regulation (eu) 2017/745. Creating a declaration of conformity (doc) is an essential step for manufacturers of medical devices and in vitro diagnostic devices who wish to enter the european union market. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) Eu declaration of conformity (sample) 1. Product model/product (product, type, batch or serial number):. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. Standards used for demonstration of compliance: Object of the declaration (identification of product allowing traceability. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) Additional information to be mentioned on. The generic template of the (eu) declaration of conformity is given in annex iii of decision 768/2008/ec. As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu doc. Pressure equipment direc˚ve (ped) 2014/68/eu. How to draft the eu declaration of conformity. Product model/product. Pressure equipment direc˚ve (ped) 2014/68/eu. Eu declaration of conformity (sample) 1. It may include a colour image of sufficient clarity to enable the identification of the product, where appropriate.) These products bear the ce mark indicating conformity with the provisions of these directives. Additional information to be mentioned on the doc may be required by. In this blog you will be guided through the process of creating a fully compliant declaration of conformity (doc), so you will be able to compile this document yourself. Standards used for demonstration of compliance: How to draft the eu declaration of conformity. This document is an eu declaration of conformity template. Object of the declaration (identification of product allowing traceability. As our customers report, amazon or the german federal network agency (bundesnetzagentur) are increasingly requesting the eu declaration of conformity from. It contains 8 points that a manufacturer must fill out to declare that a product meets relevant eu legislation and. As a general requirement, the manufacturer of medical device or accessory in compliance with the applicable requirements of mdr 2017/745 must declare the conformity with an eu doc. This object is in conformity with the following union harmonisation legislation: Product model/product (product, type, batch or serial number): (further) the following harmonised standards and.DS80D EU DECLARATION OF CONFORMITY EN
EU Declaration of Conformity Fasetech
A Guide to CE Marking EU Authorised Rep Compliance
EU Declaration of Conformity
The EU declaration of conformity F2 Tech Notes
Declaration Of Conformity Template
EU Declaration of Conformity
Eu Declaration Of Conformity Template
Fillable Eu Declaration Of Conformity (Doc) printable pdf download
EC Declaration of Conformity
Essential Guide To Ensure Compliance And Meet New Standards.
The Generic Template Of The (Eu) Declaration Of Conformity Is Given In Annex Iii Of Decision 768/2008/Ec.
The Eu Declaration Of Conformity Previously Was Called An ‘Ec Declaration Of Conformity’.
Comply With Medical Device Regulation (Eu) 2017/745.
Related Post: