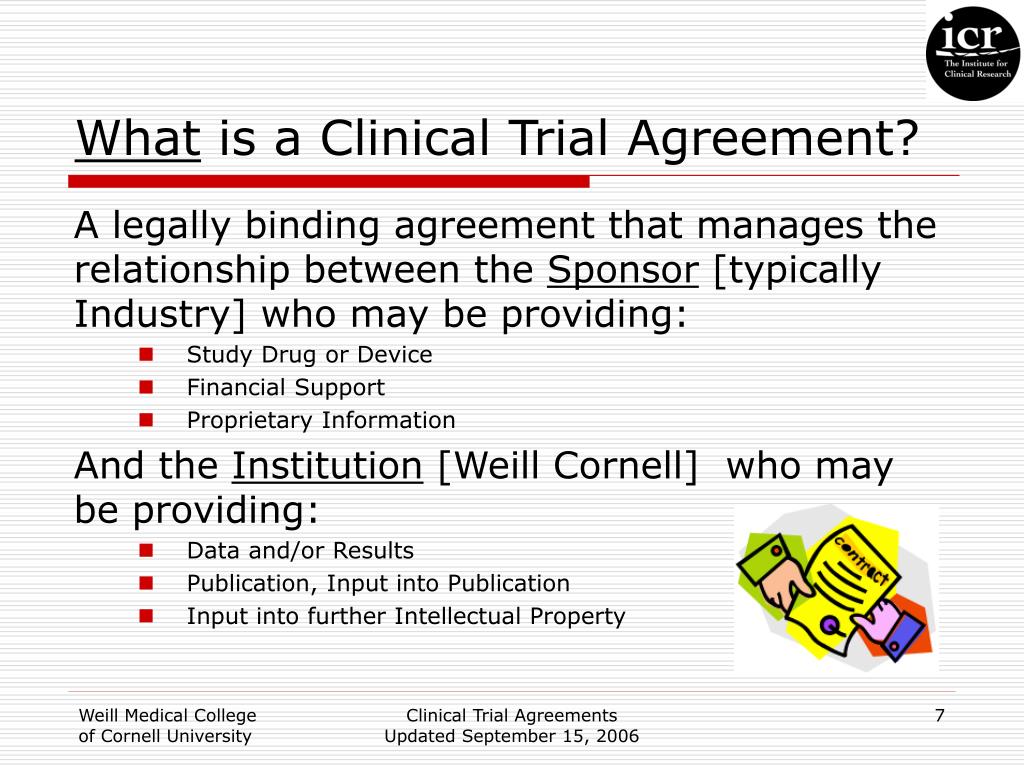
Clinical Study Agreement Template
Clinical Study Agreement Template - They provide a framework for conducting the trial in line with. In this agreement, the following capitalized words and phrases have the following meanings: Whereas, the study contemplated by this agreement is of mutual interest and benefit to institution and sponsor, and will further the instructional and research objectives of institution. This clinical trial agreement is between a university and a sponsor. • the regents of the university of california on behalf of its. 1.1 ski/memorial will perform for company a clinical trial entitled “_____” (hereinafter “study”). Download or preview 7 pages of pdf version of clinical study agreement template (doc: The purpose of a description in a clinical trial agreement is to clearly state the study’s. This early feasibility study (“efs”) master clinical trial agreement template is provided by the medical device. Clinical research study set forth in the research protocol developed by the sponsor dated _______________ and entitled _______________________________________________. Download or preview 7 pages of pdf version of clinical study agreement template (doc: They provide a framework for conducting the trial in line with. • the regents of the university of california on behalf of its. Clinical research study set forth in the research protocol developed by the sponsor dated _______________ and entitled _______________________________________________. Efs master clinical study agreement important note: Developing a comprehensive clinical trial protocol. Here are the essential elements that should be included in a clinical trial agreement: Whereas, the study contemplated by this agreement is of mutual interest and benefit to institution and company, and will further the instructional and research objectives of institution. Clinical trial agreements set out how the clinical trial will be conducted, as well as the roles and responsibilities of each party. The purpose of a description in a clinical trial agreement is to clearly state the study’s. Clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and guaranteeing that regulatory requirements are met. The translational research coordinator (trc) will support the gastrointestinal (gi) oncology translational research program in the areas of patient consenting, sample. • the regents of the university of california on behalf of its. Download free clinical. Developing a comprehensive clinical trial protocol. The protocol is the backbone of your clinical trial, detailing every step of the study. They provide a framework for conducting the trial in line with. Efs master clinical study agreement important note: Whereas, the study contemplated by this agreement is of mutual interest and benefit to institution and company, and will further the. 1.1 ski/memorial will perform for company a clinical trial entitled “_____” (hereinafter “study”). The translational research coordinator (trc) will support the gastrointestinal (gi) oncology translational research program in the areas of patient consenting, sample. Efs master clinical study agreement important note: This clinical study agreement (“agreement”) is made and entered into as of mmm dd, yyyy (“effective date”) by and. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Clinical research study set forth in the research protocol developed by the sponsor dated _______________ and entitled _______________________________________________. Whereas, the study contemplated by this agreement is of mutual interest and benefit to institution and company, and will. 1.1 “ agreement ” means this clinical trial agreement, including the attached appendices, as. Clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and guaranteeing that regulatory requirements are met. • the regents of the university of california on behalf of its. This early feasibility study (“efs”) master clinical trial agreement template. Efs master clinical study agreement important note: The clinical trial agreement (“agreement”) is made as of the later of the signatures below (“effective date”) by and between: This clinical trial agreement is between a university and a sponsor. Clinical research study set forth in the research protocol developed by the sponsor dated _______________ and entitled _______________________________________________. Whereas, the study contemplated. This clinical trial agreement is between a university and a sponsor. Whereas, sponsor desires institution to study the safety and/or efficacy of [drug or device] (“study drug”) or (“study device”) and institution is willing to perform a clinical study of the. In this agreement, the following capitalized words and phrases have the following meanings: Clinical trial agreements are vital for. The purpose of a description in a clinical trial agreement is to clearly state the study’s. Clinical trial agreements set out how the clinical trial will be conducted, as well as the roles and responsibilities of each party. 1.2 the study under this agreement will be conducted under the norms of form fda. You can open the clinical trial agreement. 1.1 “ agreement ” means this clinical trial agreement, including the attached appendices, as. • the regents of the university of california on behalf of its. Clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and guaranteeing that regulatory requirements are met. It ensures consistency across clinical trial. This early feasibility study. For cases where standard contracts are not supplied by sponsors, the clinical contracts division (ccd) has the following agreement templates available: Download or preview 7 pages of pdf version of clinical study agreement template (doc: 95.1 kb ) for free. Whereas, sponsor desires institution to study the safety and/or efficacy of [drug or device] (“study drug”) or (“study device”) and. Download or preview 7 pages of pdf version of clinical study agreement template (doc: The protocol is the backbone of your clinical trial, detailing every step of the study. The purpose of a description in a clinical trial agreement is to clearly state the study’s. Clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and guaranteeing that regulatory requirements are met. Efs master clinical study agreement important note: Whereas, sponsor desires institution to study the safety and/or efficacy of [drug or device] (“study drug”) or (“study device”) and institution is willing to perform a clinical study of the. Easiest setup experiencefree mobile appfast, easy & secure It ensures consistency across clinical trial. Developing a comprehensive clinical trial protocol. 1.1 “ agreement ” means this clinical trial agreement, including the attached appendices, as. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. 1.2 the study under this agreement will be conducted under the norms of form fda. 95.1 kb ) for free. This early feasibility study (“efs”) master clinical trial agreement template is provided by the medical device. Welcome to global health trials' tools and templates library. The translational research coordinator (trc) will support the gastrointestinal (gi) oncology translational research program in the areas of patient consenting, sample.24+ SAMPLE Clinical Trial Agreement in PDF Doc Template pdfFiller
Clinical Trial Agreement Template
Clinical study agreement template in Word and Pdf formats
Accelerated Clinical Trial Agreement
WA Health MA Clinical Trial Research Agreement Doc Template pdfFiller
Investigator Initiated Clinical Trial Agreement Template
WA Health Clinical Trial Research Agreement
TEMPLATE CLINICAL TRIAL AGREEMENT
Clinical study agreement template in Word and Pdf formats page 7 of 7
Clinical study agreement template in Word and Pdf formats
The Clinical Trial Agreement (“Agreement”) Is Made As Of The Later Of The Signatures Below (“Effective Date”) By And Between:
They Provide A Framework For Conducting The Trial In Line With.
1.1 Ski/Memorial Will Perform For Company A Clinical Trial Entitled “_____” (Hereinafter “Study”).
Whereas, The Study Contemplated By This Agreement Is Of Mutual Interest And Benefit To Institution And Company, And Will Further The Instructional And Research Objectives Of Institution.
Related Post: