Clinical Project Manager Templates Checklist For New Protocol
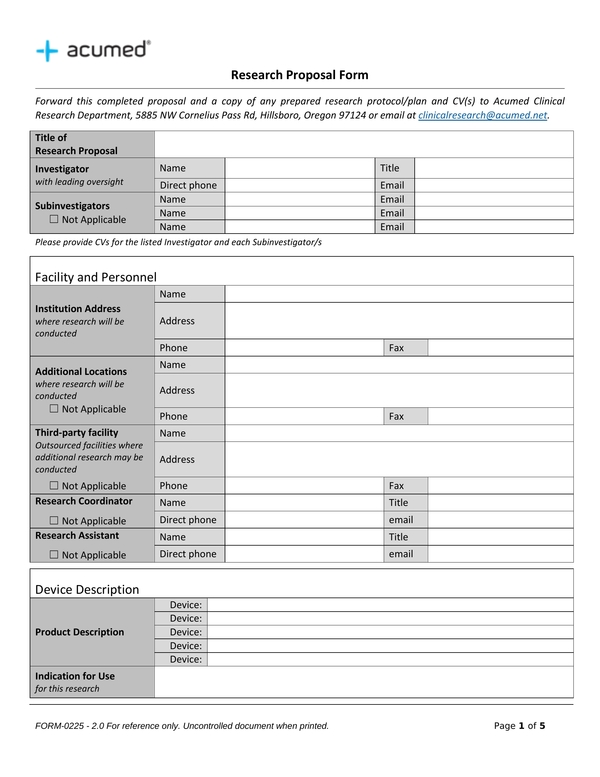
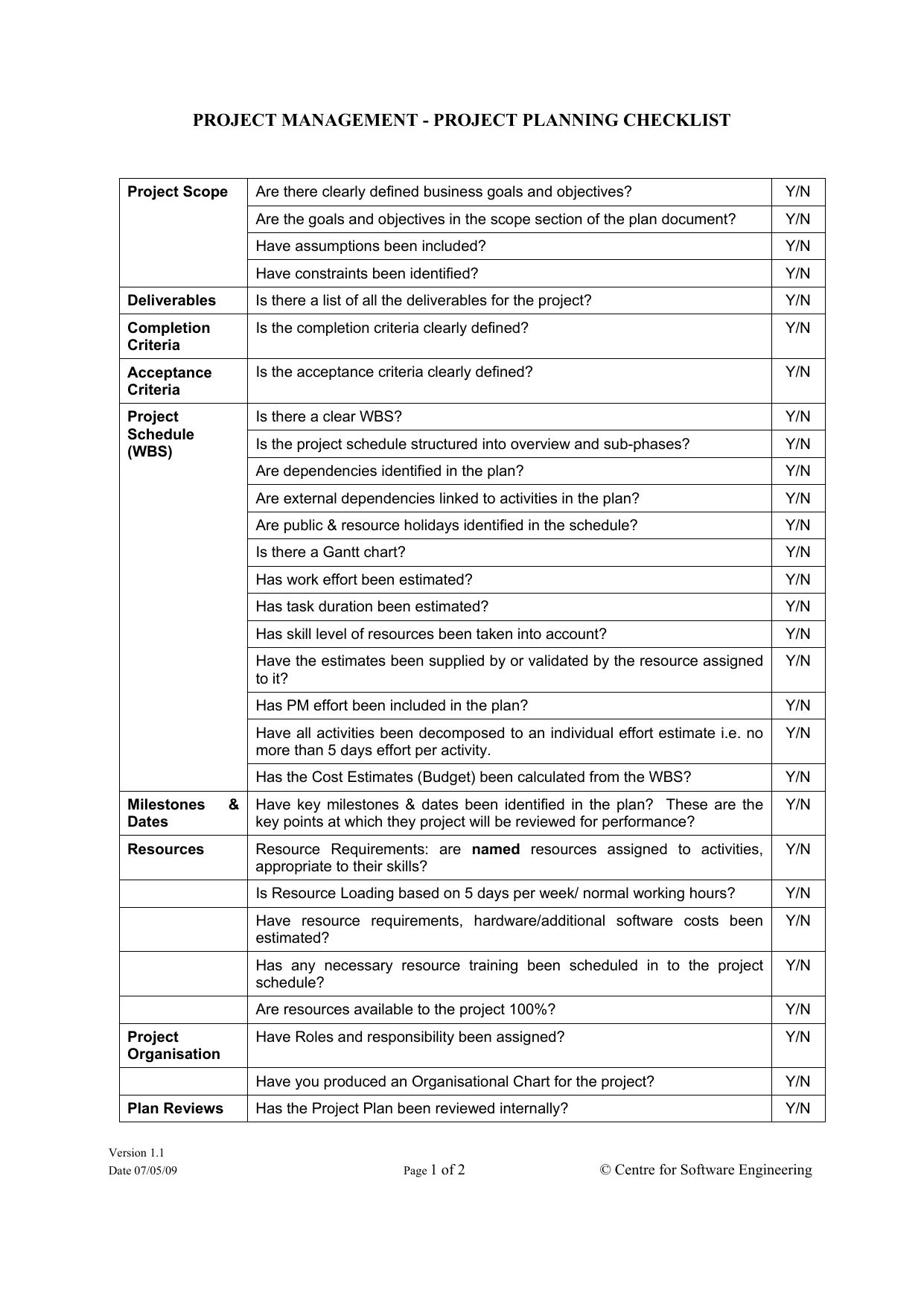
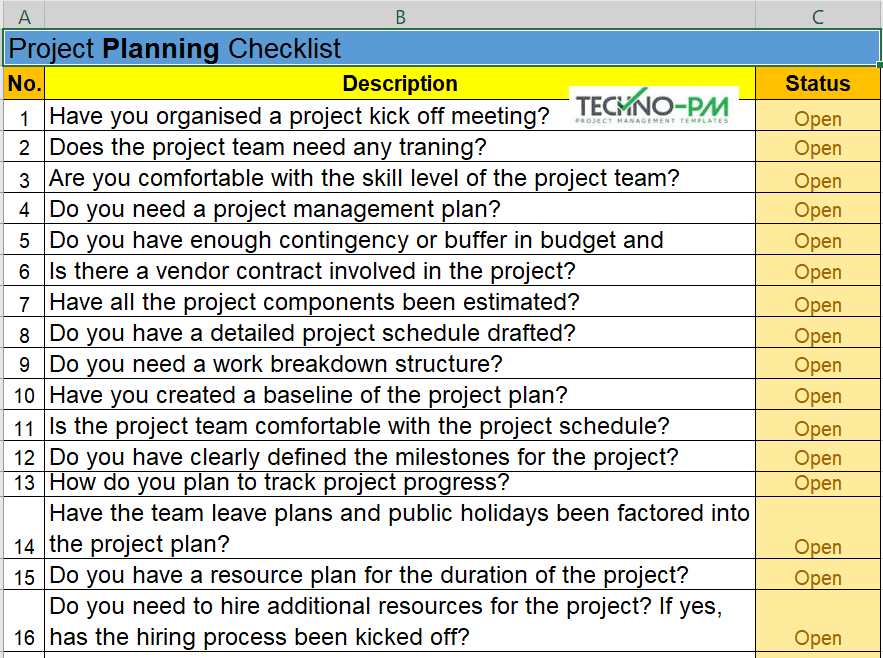
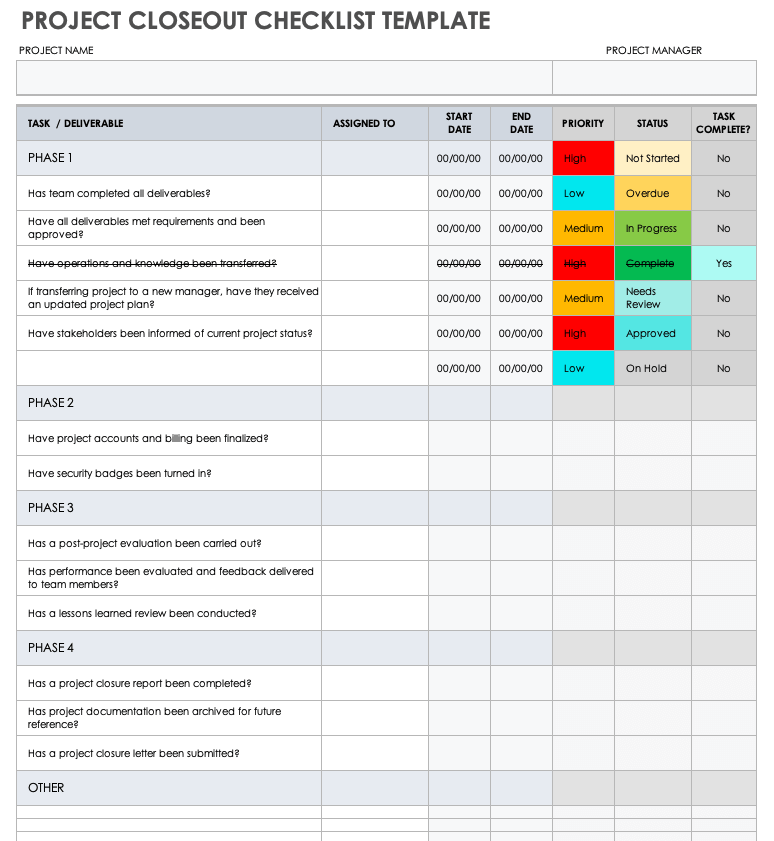
Clinical Project Manager Templates Checklist For New Protocol - This section includes a sample checklist of potential trial. During the planning and implementation of an epct, pms will be responsible for managing a large amount of documentation. Healthcare project management refers to the organization, planning, and execution of a particular project and its resources. Being a clinical project manager is no small feat. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic,. This article will provide a comprehensive checklist designed specifically for clinical project managers embarking on new protocols, covering all essential aspects from initial. This template helps you embrace flexibility and promote collaboration with all stakeholders. Template to construct a new study budget or use as a guide for existing study budget spreadsheets. This clinical trial project management plan template is a comprehensive tool for project managers and teams to plan and execute successful clinical trials. Site capabilities highlight frequently used resources; Below is a detailed training checklist for study nurses involved in clinical trials: Give a more holistic history of the project’s evolution, provide project continuity, and better orient stakeholders and end users to the. This template helps you embrace flexibility and promote collaboration with all stakeholders. The three steps to reviewing a clinical trial protocol are assessing the essential sections, reviewing the entire protocol, and reading the informed consent template. Template for writing a research protocol involving participants. Welcome to global health trials' tools and templates library. Clinical monitoring and data management plan (cmp/dmp) checklist. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Streamline processes for every phase of the trial. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic,. With these three templates, you can: Share team news and announcements; Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Clinical monitoring and data management plan (cmp/dmp) checklist. This clinical trial project management plan template is a comprehensive tool for project managers and teams to plan. During the planning and implementation of an epct, pms will be responsible for managing a large amount of documentation. This article will provide a comprehensive checklist designed specifically for clinical project managers embarking on new protocols, covering all essential aspects from initial. Niams has guidelines and templates to help investigators develop a study mop. Being a clinical project manager is. Below is a detailed training checklist for study nurses involved in clinical trials: With these three templates, you can: The project management life cycle for clinical trials is comprised of: Welcome to global health trials' tools and templates library. Clinical monitoring and data management plan (cmp/dmp) checklist. Welcome to global health trials' tools and templates library. This article will provide a comprehensive checklist designed specifically for clinical project managers embarking on new protocols, covering all essential aspects from initial. Give a more holistic history of the project’s evolution, provide project continuity, and better orient stakeholders and end users to the. With these three templates, you can: Below. This clinical trial project management plan template is a comprehensive tool for project managers and teams to plan and execute successful clinical trials. You are approached by your supervisor or department to be the project manager for an upcoming. If you are a seasoned project manager involved in clinical research, this activity list with the typical activities performed during the. This section includes a sample checklist of potential trial. Send due date reminders using the work progress tracker template with automation features Template to construct a new study budget or use as a guide for existing study budget spreadsheets. With these three templates, you can: Please note that this page has been updated for 2015 following a quality check and. Niams has guidelines and templates to help investigators develop a study mop. Being a clinical project manager is no small feat. Template for writing a research protocol involving participants. Site capabilities highlight frequently used resources; Below is a detailed training checklist for study nurses involved in clinical trials: This article will provide a comprehensive checklist designed specifically for clinical project managers embarking on new protocols, covering all essential aspects from initial. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. This section includes a sample checklist of potential trial. Share team news and announcements;. With these three templates, you can: Template to construct a new study budget or use as a guide for existing study budget spreadsheets. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Site capabilities highlight frequently used resources; Below is a detailed training checklist for study. Below is a detailed training checklist for study nurses involved in clinical trials: You are approached by your supervisor or department to be the project manager for an upcoming. This article will provide a comprehensive checklist designed specifically for clinical project managers embarking on new protocols, covering all essential aspects from initial. The three steps to reviewing a clinical trial. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Send due date reminders using the work progress tracker template with automation features This section includes a sample checklist of potential trial. During the planning and implementation of an epct, pms will be responsible for managing a large amount of documentation. Streamline processes for every phase of the trial. Site capabilities highlight frequently used resources; Template to construct a new study budget or use as a guide for existing study budget spreadsheets. Niams has guidelines and templates to help investigators develop a study mop. The three steps to reviewing a clinical trial protocol are assessing the essential sections, reviewing the entire protocol, and reading the informed consent template. Being a clinical project manager is no small feat. Healthcare project management refers to the organization, planning, and execution of a particular project and its resources. If you are a seasoned project manager involved in clinical research, this activity list with the typical activities performed during the clinical trial will be a great reminder and a good starting point in. Template for writing a research protocol involving participants. You are approached by your supervisor or department to be the project manager for an upcoming. Clinical monitoring and data management plan (cmp/dmp) checklist. Share team news and announcements;Clinical Trial Project Management Plan Template
Clinical Trial Project Management Plan Template
Download Project Checklist Template Excel Pdf Rtf Word with
Project Management Checklist Excel Template Project Management
The Ultimate Project Documentation Checklist Ensuring Success Through
Free Clinical Trial Templates Smartsheet
Project Management Checklist Excel Template Project Management
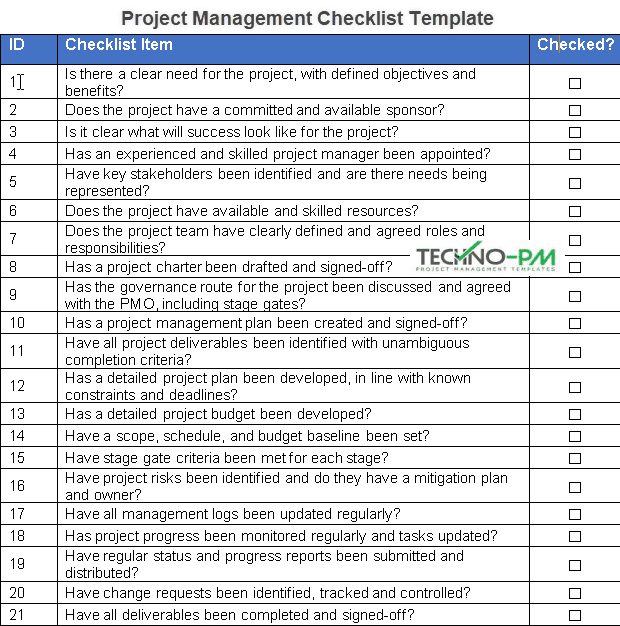
Project Management Checklist Template Free
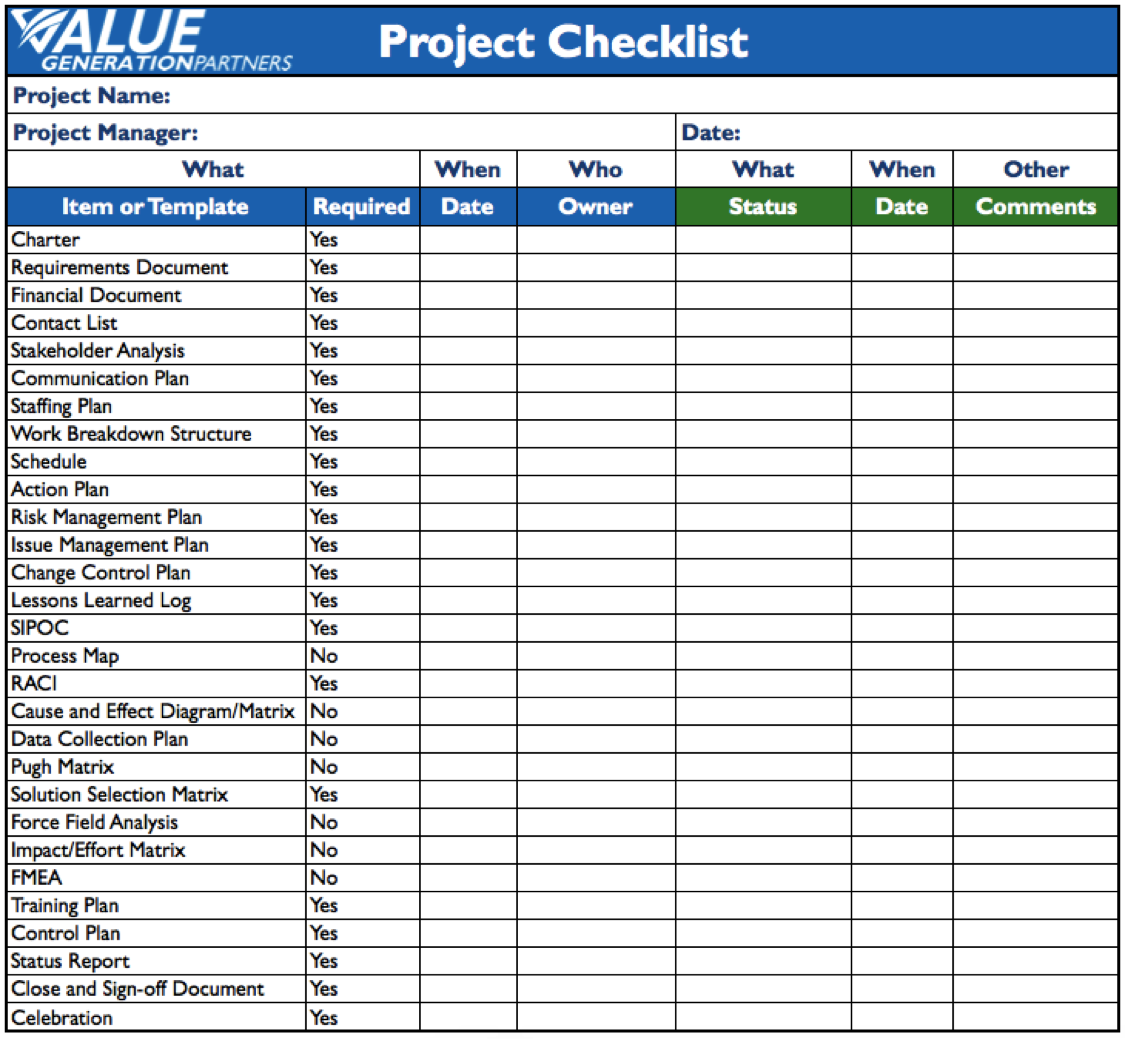
project management checklist template Generating Value by Using a
Clinical Trial Project Management Plan Template
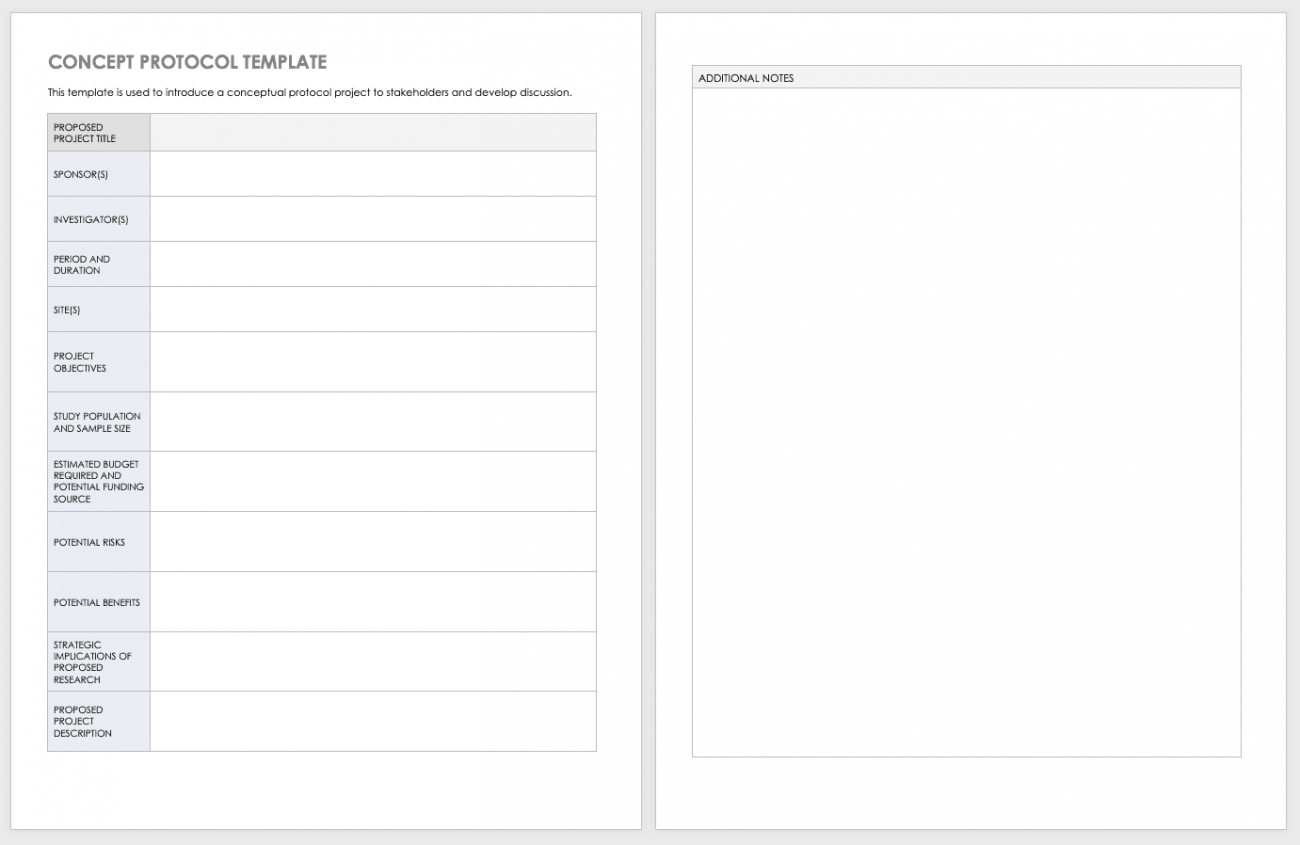
This Article Will Provide A Comprehensive Checklist Designed Specifically For Clinical Project Managers Embarking On New Protocols, Covering All Essential Aspects From Initial.
This Template Helps You Embrace Flexibility And Promote Collaboration With All Stakeholders.
Below Is A Detailed Training Checklist For Study Nurses Involved In Clinical Trials:
Clinical Trial Protocol Template This Protocol Template Is Designed To Help Research Teams Develop A Clinical Trial Protocol That Includes An Investigational Intervention (Drug, Biologic,.
Related Post: