Cap Tumor Template
Cap Tumor Template - View current protocols and download templates. Completion of the template is the responsibility of the. The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports within their current system workflows. For reporting molecular testing and immunohistochemistry for mismatch repair proteins, and for other cancer biomarker testing results, the cap colorectal biomarker template should be. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient… This allows anatomical pathologists to report on a far greater number of cases while. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Cancer biomarker testing results, the cap colorectal biomarker template should be used. The list of mandated cap cancer checklists and effective dates can be found in appendix 4.1. These updated ecc's include new fields, terms, options, titles, and even new templates to be used. From the college of american pathologists, american society for clinical pathology, and american society of clinical oncology. View current protocols and download templates. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Read more about the cap notice regarding. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. To promote understanding of the college of american pathologists (cap) cancer protocols and their critical use in cancer patient care, and advance collaboration and. This allows anatomical pathologists to report on a far greater number of cases while. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. Completion of the template is the responsibility of the. The html form and cascade style sheet were created on pform , with references to javascripts and. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. For reporting molecular testing and immunohistochemistry for mismatch repair proteins, and for other cancer biomarker testing results, the cap colorectal biomarker template should be. The electronic versions of over 100 cancer protocols. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. From the college of american pathologists, american society for clinical pathology, and american society of clinical oncology. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. These updated ecc's include new fields, terms,. From the college of american pathologists, american society for clinical pathology, and american society of clinical oncology. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Access the new autopsy protocol; Cancer biomarker testing results, the cap colorectal biomarker template should be used. Completion. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. To promote understanding of the college of american pathologists (cap) cancer protocols and their critical use in cancer patient care, and advance collaboration and. The list of mandated cap cancer checklists and effective dates can. Findings revealed that most of the approximately 160 respondents (87 percent) use the cap cancer protocols, four percent use the international collaboration on cancer reporting (iccr). These updated ecc's include new fields, terms, options, titles, and even new templates to be used. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete reporting of malignant tumors. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. To date, cco pathology laboratory medicine program mandated use of 6 cap biomarker. To promote understanding of the college of american pathologists (cap) cancer protocols and their critical use in cancer patient care, and advance collaboration and. The list of mandated cap cancer checklists and effective. Access the new autopsy protocol; Pending biomarker studies should be listed in the comments section of this report. To promote understanding of the college of american pathologists (cap) cancer protocols and their critical use in cancer patient care, and advance collaboration and. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete reporting of malignant tumors. Pending biomarker studies should be listed in the comments section of this report. Completion of the template is the responsibility of the. The list of mandated cap cancer checklists and effective dates can be found in appendix 4.1. From the college of american pathologists, american society for clinical pathology, and american society of clinical oncology. For accreditation purposes, only the. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. For reporting molecular testing and immunohistochemistry for mismatch repair proteins, and for other cancer biomarker testing results, the cap colorectal biomarker. To date, cco pathology laboratory medicine program mandated use of 6 cap biomarker. To promote understanding of the college of american pathologists (cap) cancer protocols and their critical use in cancer patient care, and advance collaboration and. For reporting molecular testing and immunohistochemistry for mismatch repair proteins, and for other cancer biomarker testing results, the cap colorectal biomarker template should be. Read more about the cap notice regarding. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Findings revealed that most of the approximately 160 respondents (87 percent) use the cap cancer protocols, four percent use the international collaboration on cancer reporting (iccr). View current protocols and download templates. Pending biomarker studies should be listed in the comments section of this report. The list of mandated cap cancer checklists and effective dates can be found in appendix 4.1. From the college of american pathologists, american society for clinical pathology, and american society of clinical oncology. The html form and cascade style sheet were created on pform , with references to javascripts and. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. These updated ecc's include new fields, terms, options, titles, and even new templates to be used. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. Cancer biomarker testing results, the cap colorectal biomarker template should be used. For reporting cancer biomarker testing results, the cap lung biomarker template may be used.Cap Cancer Template
Cap Cancer Template
Cap Cancer Templates
Cap Cancer Templates
Cap Cancer Templates
Cap Cancer Template prntbl.concejomunicipaldechinu.gov.co
Cap Protocol Templates
CAP Cancer Protocol Intrahepatic Bile Ducts Doc Template pdfFiller
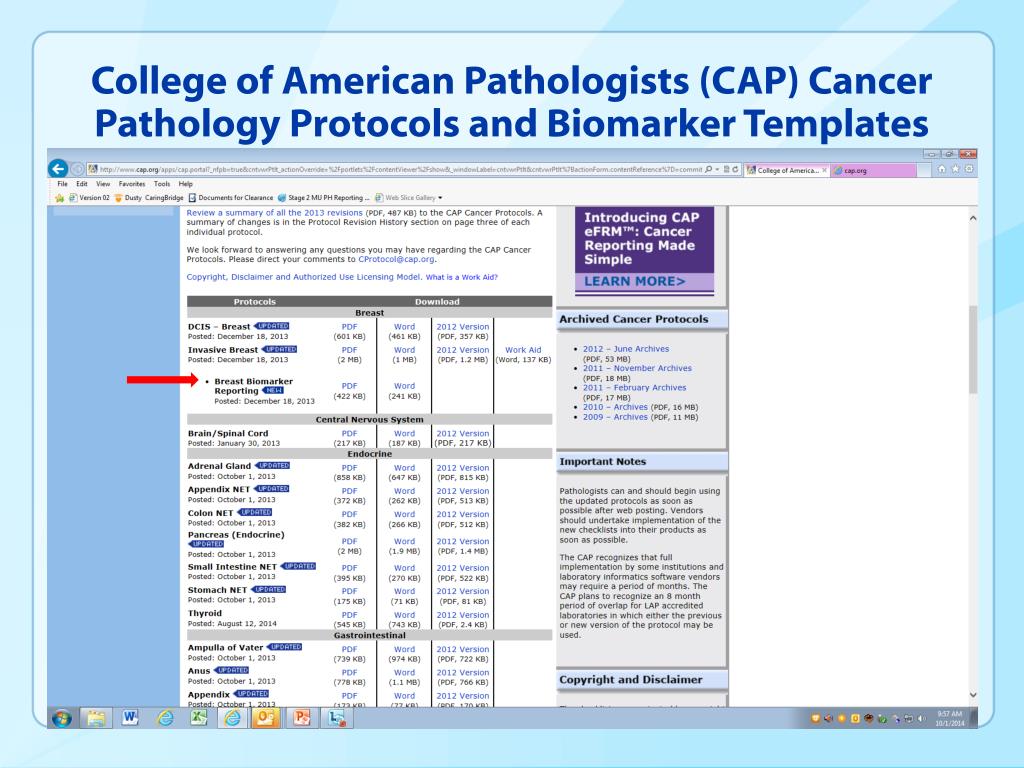
PPT Cancer Pathology and Biomarker Reporting PowerPoint Presentation
CAP Cancer Protocol Esophagus Doc Template pdfFiller
Cancer Protocols And Biomarker Reporting Templates;
Cap Cancer Protocol Templates Provide Guidelines For Collecting The Essential Data Elements For Complete Reporting Of Malignant Tumors And Optimal Patient…
This Allows Anatomical Pathologists To Report On A Far Greater Number Of Cases While.
Access The New Autopsy Protocol;
Related Post: