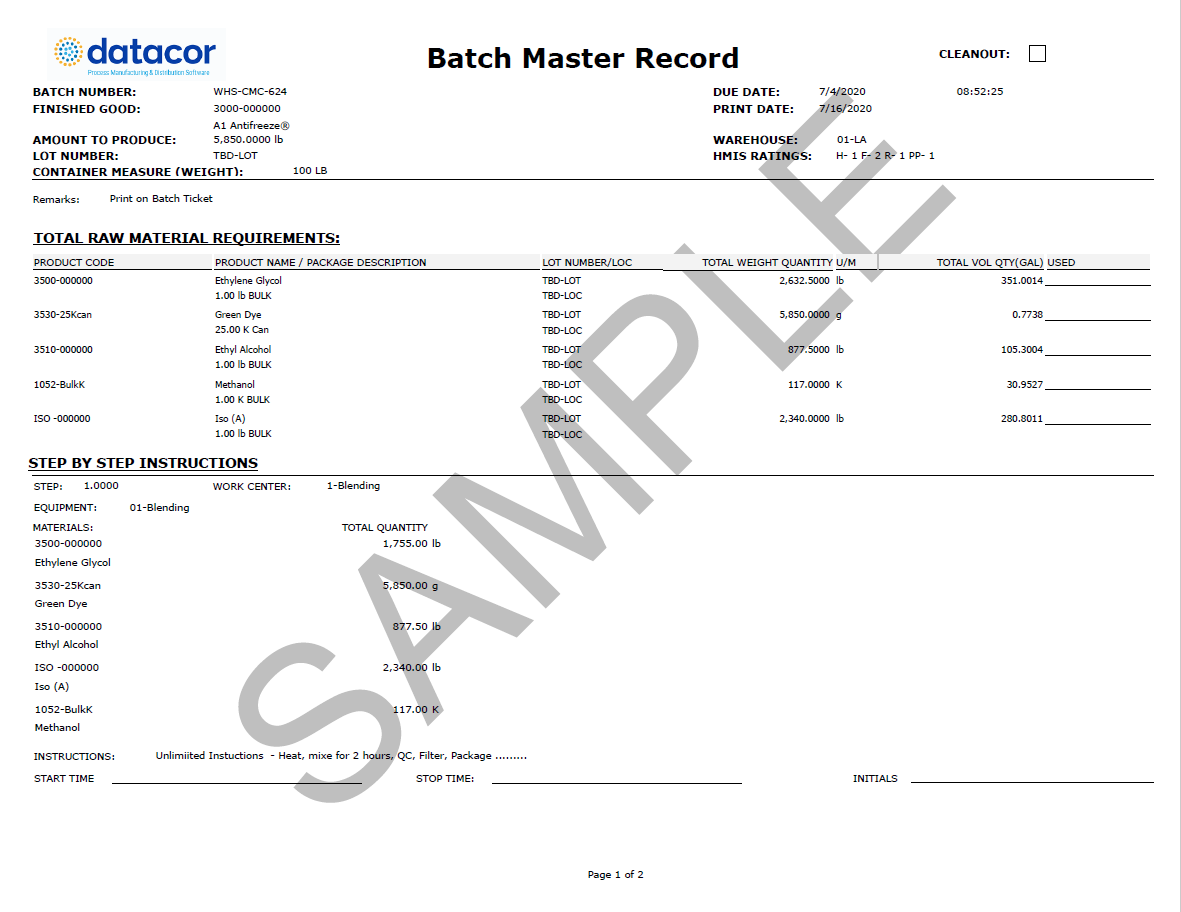
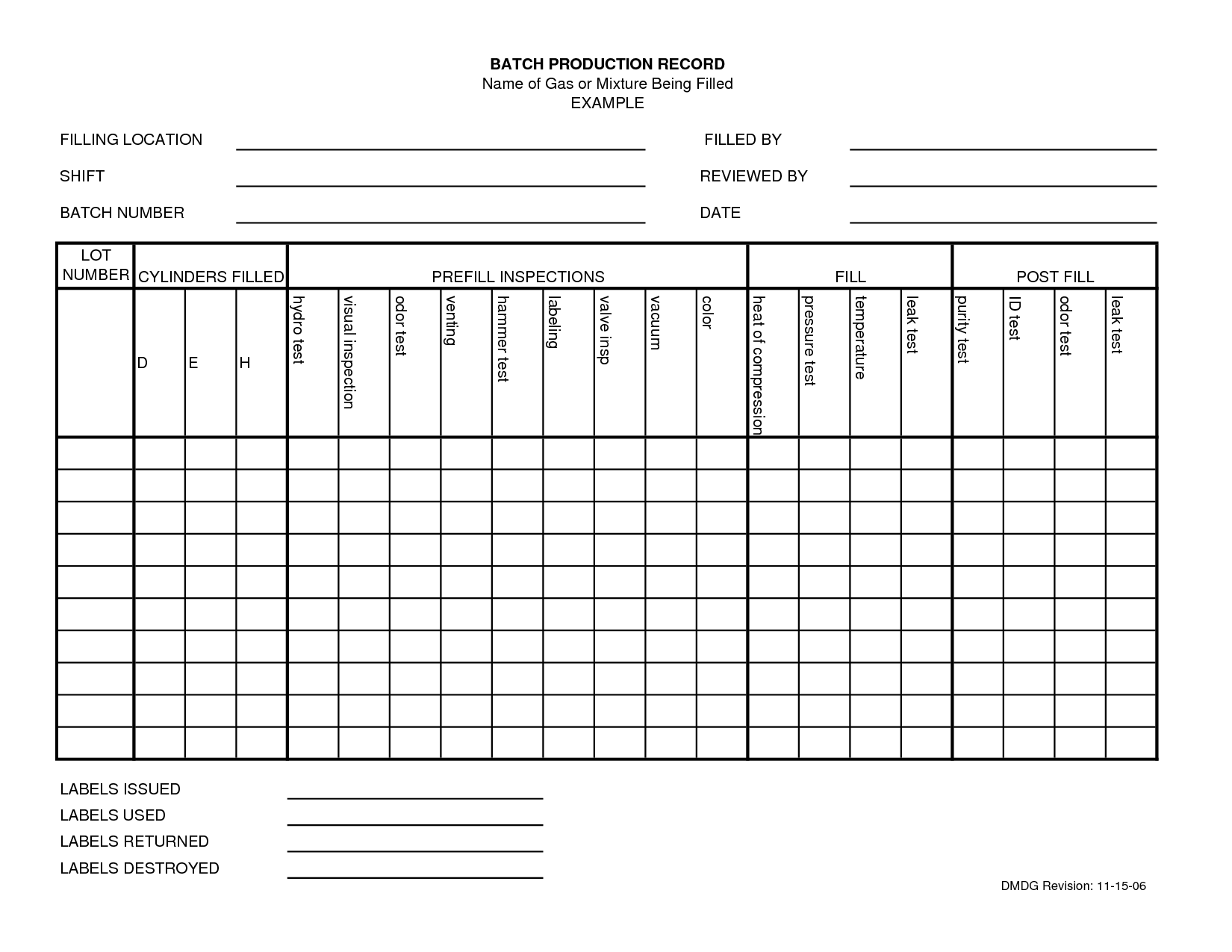
Batch Record Template
Batch Record Template - The device master record should list all of the documents and procedures used to make the product. Qa compliance 10th november 2010 10:32 am re: The device history record is usually a folder that contains (at least in our medical device plant): The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. Further i want some guidance in designing batch manufacturing / processing record for injection molding machines for different parts used for syringe manufacturing. If any one can guide or discuss with me the batch number allocation system of their manufacturing unit for injection molding department, will b e highly appreciated. Jun 3, 2011 #2 vxmartinez said: I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). Bmr is specific to a manufacturing location, batch size; Batch and master batch record template for pharmaceutical industry. The device master record should list all of the documents and procedures used to make the product. * either a copy of the documents the product was made to or a traveler that lists the documents, revisions, and dates of manufacture. Further i want some guidance in designing batch manufacturing / processing record for injection molding machines for different parts used for syringe manufacturing. Bmr is specific to a manufacturing location, batch size; I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). Batch and master batch record template for pharmaceutical industry. The device history record is usually a folder that contains (at least in our medical device plant): Jun 3, 2011 #2 vxmartinez said: That is what i would have said. Qa compliance 10th november 2010 10:32 am re: The device history record is usually a folder that contains (at least in our medical device plant): I have a batch record template i can share. Batch and master batch record template for pharmaceutical industry. The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. I am looking. * either a copy of the documents the product was made to or a traveler that lists the documents, revisions, and dates of manufacture. Batch and master batch record template for pharmaceutical industry. These requirements are defined in 21cfr 820.3(i) and 21cfr 820.86. I am looking for templates of master production record (per 21 cfr part 211.186), and master batch. The device history record is usually a folder that contains (at least in our medical device plant): Master batch record, probably refers (i say prob cause not a term i am familiar with) to the controlled copy of a batch record held in an area that copies are made from for use when you manufacture, when there is an update. The device master record should list all of the documents and procedures used to make the product. * either a copy of the documents the product was made to or a traveler that lists the documents, revisions, and dates of manufacture. It derived based on the master formula record. Batch and master batch record template for pharmaceutical industry. Master batch. I have a batch record template i can share. I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). Bmr is specific to a manufacturing location, batch size; The device history record is the evidence that a particular unit, batch or lot of devices was made according. Qa compliance 10th november 2010 10:32 am re: The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. The device master record should list all of the documents and procedures used to make the product. It derived based on the master formula record. Master batch record, probably refers. Qa compliance 10th november 2010 10:32 am re: Master batch record, probably refers (i say prob cause not a term i am familiar with) to the controlled copy of a batch record held in an area that copies are made from for use when you manufacture, when there is an update to the batch record in question the current master. I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. * either a copy of the documents the product was made to or a traveler. * either a copy of the documents the product was made to or a traveler that lists the documents, revisions, and dates of manufacture. The device history record is usually a folder that contains (at least in our medical device plant): These requirements are defined in 21cfr 820.3(i) and 21cfr 820.86. If any one can guide or discuss with me. The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). I have a batch record template i can share. Master batch record, probably refers (i. Master batch record, probably refers (i say prob cause not a term i am familiar with) to the controlled copy of a batch record held in an area that copies are made from for use when you manufacture, when there is an update to the batch record in question the current master becomes superseded and is replaced with this new master. These requirements are defined in 21cfr 820.3(i) and 21cfr 820.86. I have a batch record template i can share. Qa compliance 10th november 2010 10:32 am re: It derived based on the master formula record. That is what i would have said. If any one can guide or discuss with me the batch number allocation system of their manufacturing unit for injection molding department, will b e highly appreciated. The device master record should list all of the documents and procedures used to make the product. Further i want some guidance in designing batch manufacturing / processing record for injection molding machines for different parts used for syringe manufacturing. Jun 3, 2011 #2 vxmartinez said: * either a copy of the documents the product was made to or a traveler that lists the documents, revisions, and dates of manufacture. Bmr is specific to a manufacturing location, batch size;Formula & Batch Records Wholesale Supplies Plus
Batch Record Review Checklist Template/Example
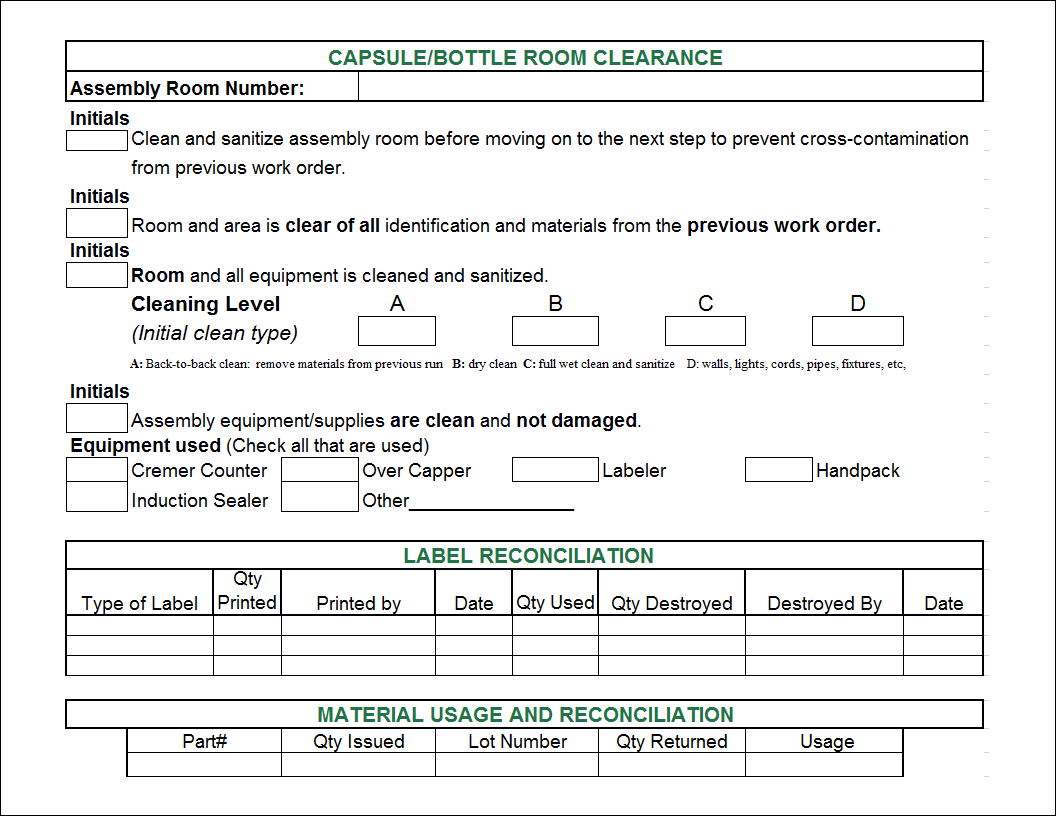
Batch Sheet Template
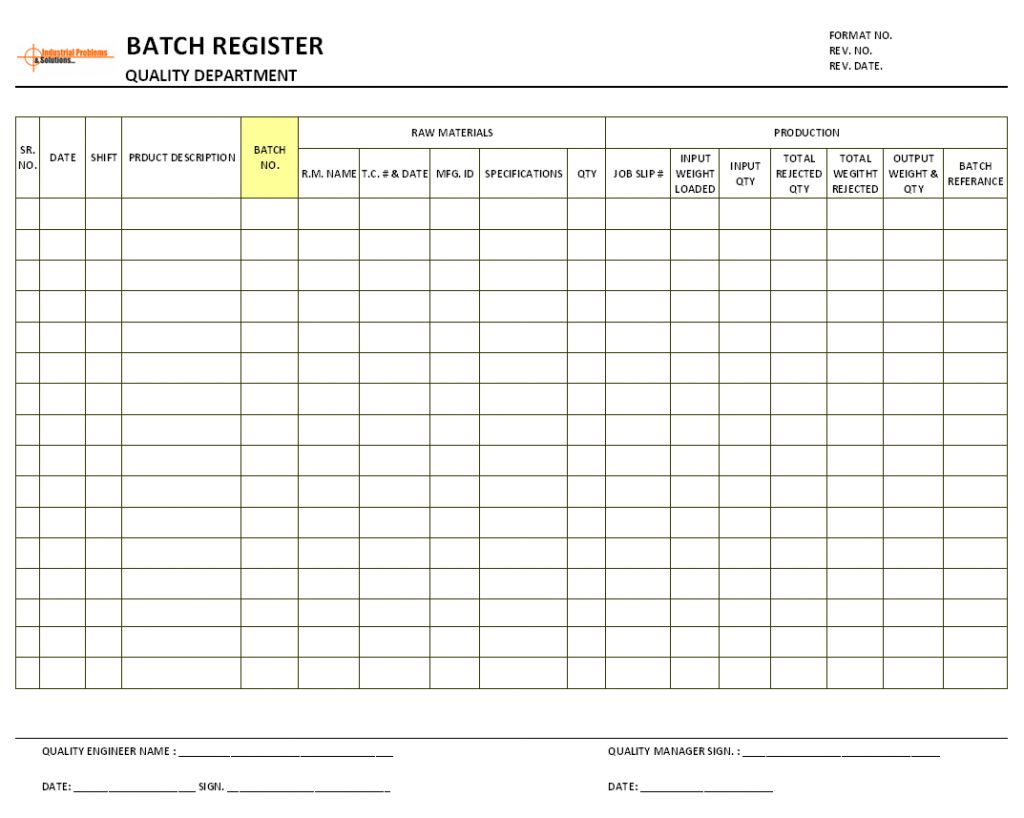
Batch Production Record Templates
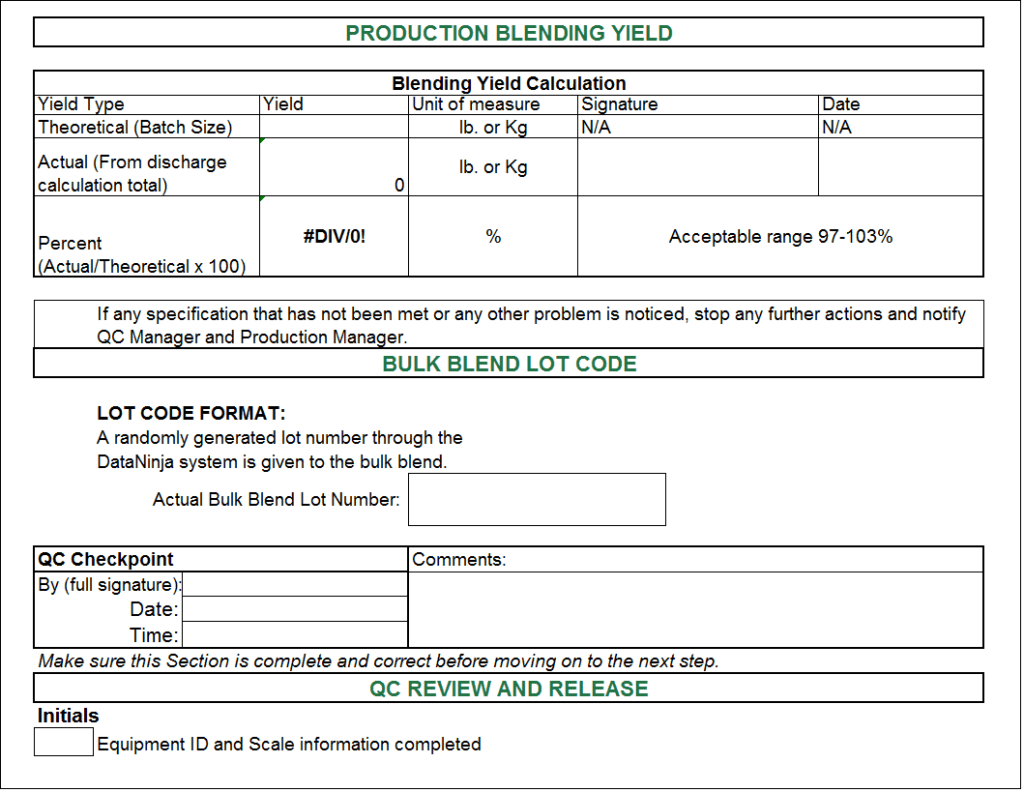
Batch Record Template Excel
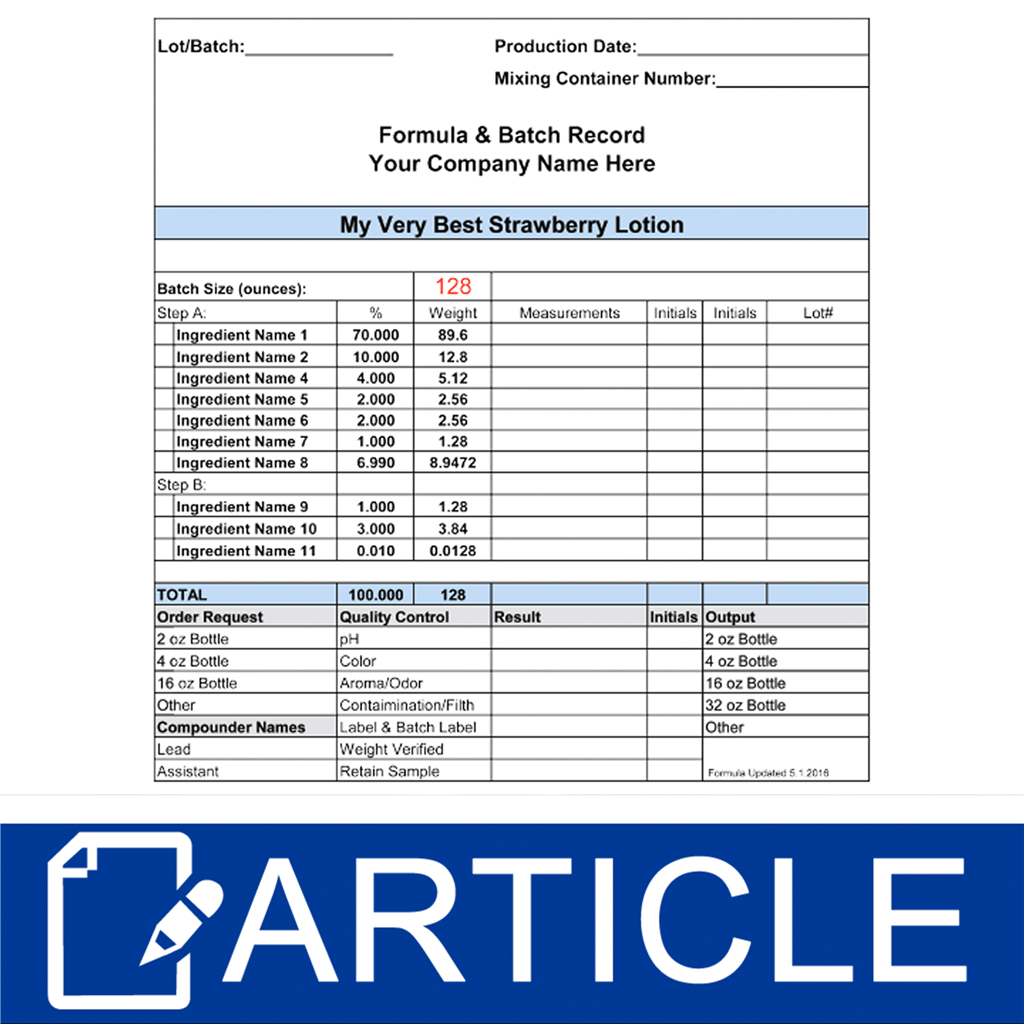
Good Manufacturing Practices Formula & Batch Records
Sample of Batch Manufacturing Record (BMR) Atorvastatin PDF
Master Batch Record Template
2024 Batch Manufacturing Record Definition, Template & Examples
Batch Sheet Template
The Device History Record Is The Evidence That A Particular Unit, Batch Or Lot Of Devices Was Made According To The Recipe.
Batch And Master Batch Record Template For Pharmaceutical Industry.
I Am Looking For Templates Of Master Production Record (Per 21 Cfr Part 211.186), And Master Batch Record (Per 21 Cfr Part 211.188).
The Device History Record Is Usually A Folder That Contains (At Least In Our Medical Device Plant):
Related Post: